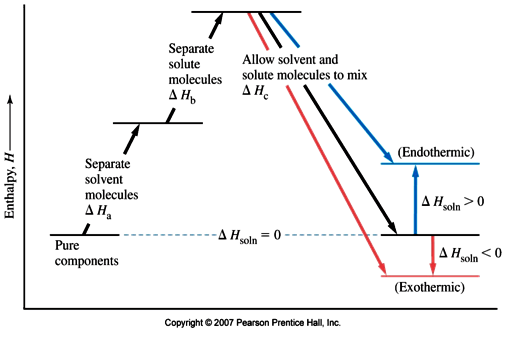
Complete The Enthalpy Diagram For An Ionic Compound Dissolving In Water
With the aid of a diagram show the enthalpy changes involved in the dissolving of an ionic compound in water where enthalpy change of solution is exothermic. Swbat to diagram and describe what occurs at the molecular level during the dissolution of an ionic compound in water a polar substance.
Co2 Enthalpy Of Sublimation Reaction And Metabolism Chem 103 Lab
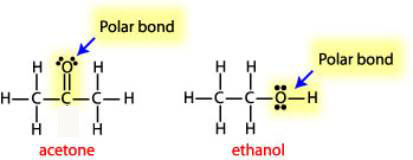
This polarity attracts and pulls apart cations and anions of an ionic compound.
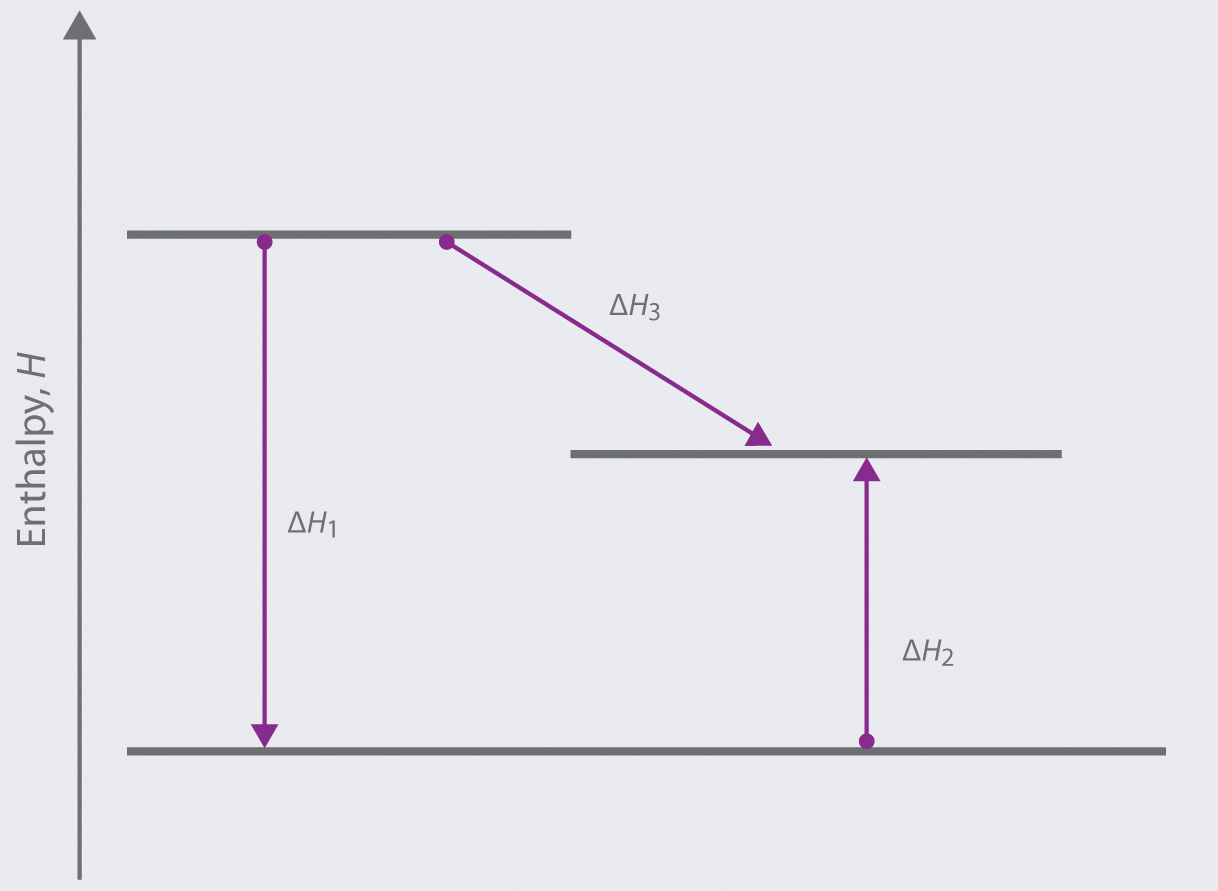
Complete the enthalpy diagram for an ionic compound dissolving in water. Ionic compounds that do dissolve in water form ions or electrolytes. The magnitude of the enthalpy of hydration is dependent on. Every compound dissolved in solution broken into ions charges everywhere.
Thus the intermolecular interactions ie ionic bonds between cenacl are broken and the salt is dissolved. Others dissolve exothermically for example naoh. Complete the enthalpy diagram for an ionic compound dissolving in water b g y g ahsolution b.
The overall chemical equation for this reaction is as follows. Enthalpies of solution may be either positive or negative in other words some ionic substances dissolved endothermically for example nacl. The enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution.
Big idea water is polar with regions of slight positive and negative charge. Acs general chemistry final study guide. The lattice energy must be supplied in order to break down a lattice and enable the ionic substance to dissolve in water.
Yg to determine the process of dissolving 1onic compounds in water is endothermic or exothermic first calculate the enthalpy of solution as follows ahsolute baq y aq the enthalpy of solutionhoattice ener. Endothermic delta h fusion delta h formation delta h solute delta h hydration exothermic delta h vaporization delta h solution this reaction is. Chem 112 acs flashcards.
Acs gen chem 1 final. The strength of the ionic attractions in the lattice is measured using the lattice energy. While cenacl dissolves in water the positive sodium cations and chloride anions are stabilized by the water molecule electric dipoles.
This energy tends to stop substances dissolving unless the energy is paid back in later. The size of the ions involved the charges on the ions. Complete the enthalpy diagram for an ionic compound dissolving in water.
Chapter 14 And 15 4 Entropy And Free Energy
Born Haber Formation Of Ionic Compounds
What Happens When A Salt Or Sugar Dissolves In Water
 Solution Chemistry Grandinetti Group
Solution Chemistry Grandinetti Group
 Acid Base Reactions Types Of Reactions Siyavula
Acid Base Reactions Types Of Reactions Siyavula
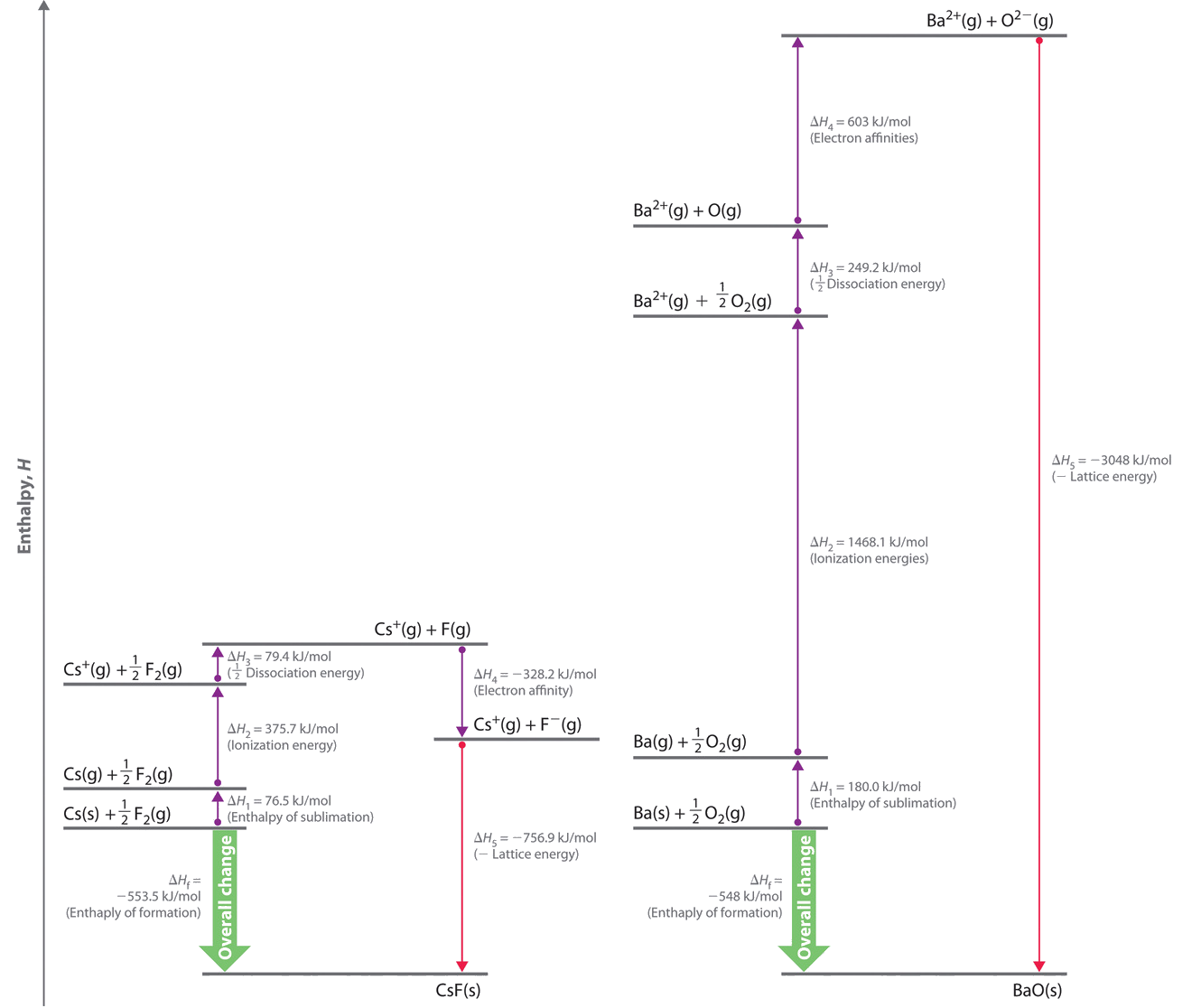 8 3 Lattice Energies In Ionic Solids Chemistry Libretexts
8 3 Lattice Energies In Ionic Solids Chemistry Libretexts
Ch150 Chapter 7 Solutions Chemistry
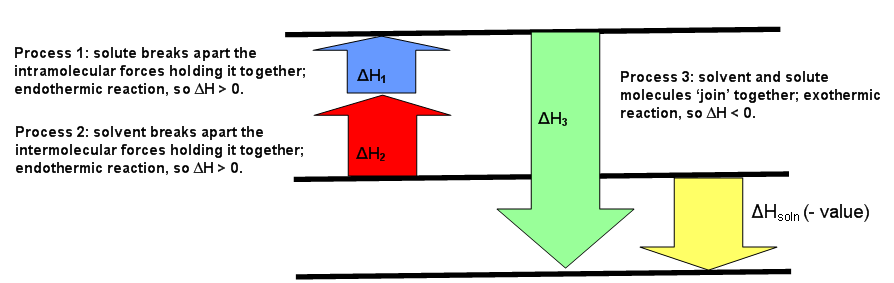 Enthalpy Of Solution Chemistry Libretexts
Enthalpy Of Solution Chemistry Libretexts
 Heat Of Neutralization Hcl Aq Naoh Aq Chemdemos
Heat Of Neutralization Hcl Aq Naoh Aq Chemdemos
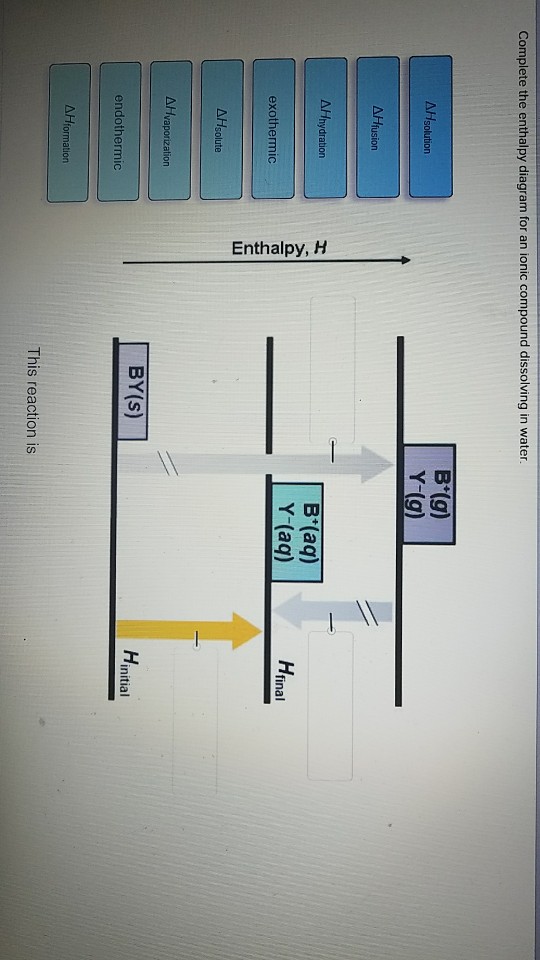
0 Response to "Complete The Enthalpy Diagram For An Ionic Compound Dissolving In Water"
Post a Comment