Sketch The Phase Diagram To Answer Whether Solid Argon Or Liquid Argon Has The Greater Density
100 11 ratings or. Since the freezing point of argon is higher than the triple point temperature the solid liquid equilibrium line slopes to the right with increasing pressure.
 57 Questions In Sublimation Science Topic
57 Questions In Sublimation Science Topic
Argon has a normal boiling point of 872 k and a melting point at 1 atm of 841 k.

Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density. Nitrogen has a normal boiling point of 773 k and a melting point at 1atm of 631 k. It has a triple point at 837 and 068. Sketch the phase diagram for nitrogen.
The liquid phase is less dense than the solid phase. It has a triple point at 837 k and 068 atmsketch the phase diagram to answer whether solid argon or liquid argon has the greater density. Does nitrogen have a stable liquid state at 1 atm.
Problem argon has a normal boiling point of 872 k and a melting point at 1 atm of 841 k. Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density. Does nitrogen has a stable liquid state at 1atm.
Its critical temperature is 1262 k an critical pressure is 255 x 104 torr. Sketch a phase diagram for nitrogen. Its critical temperature is 1508 k and critical pressure is 483 atm.
Temperatures influence a substance state for example. Chemistry 1412 2 chapter 11. Critical temperature is 1508 k and critical pressure is 483 atmit has a triple point at 837 k and 068 atm.
It has a triple point at 631 k and 940 torr. It has a triple point at 631 k and 940 torr. Solid liquid or gas state of a substance at a particular temperature is determined by their intermolecular forces.
Its critical temperature is 1508 and critical pressure is 483. Part a sketch the phase diagram to answer whether solid argon or liquid argon has the greater density. Correct exercise 1190 the high pressure phase diagram of ice is shown at the top of the next column.
It has a triple point at 837 k and 068 atmsketch the phase diagram to answer whether solid argon or liquid argon has the greater densit. Nitrogen has a normal boiling point of 773 k and a melting point at 1 atm of 631 k. Answer to nitrogen has a normal boiling point of 773 k and a melting point at 1atm of 631 k.
Gas state of a substance has the weakest intermolecular force while liquids and solids have higher intermolecular forces between the molecules.
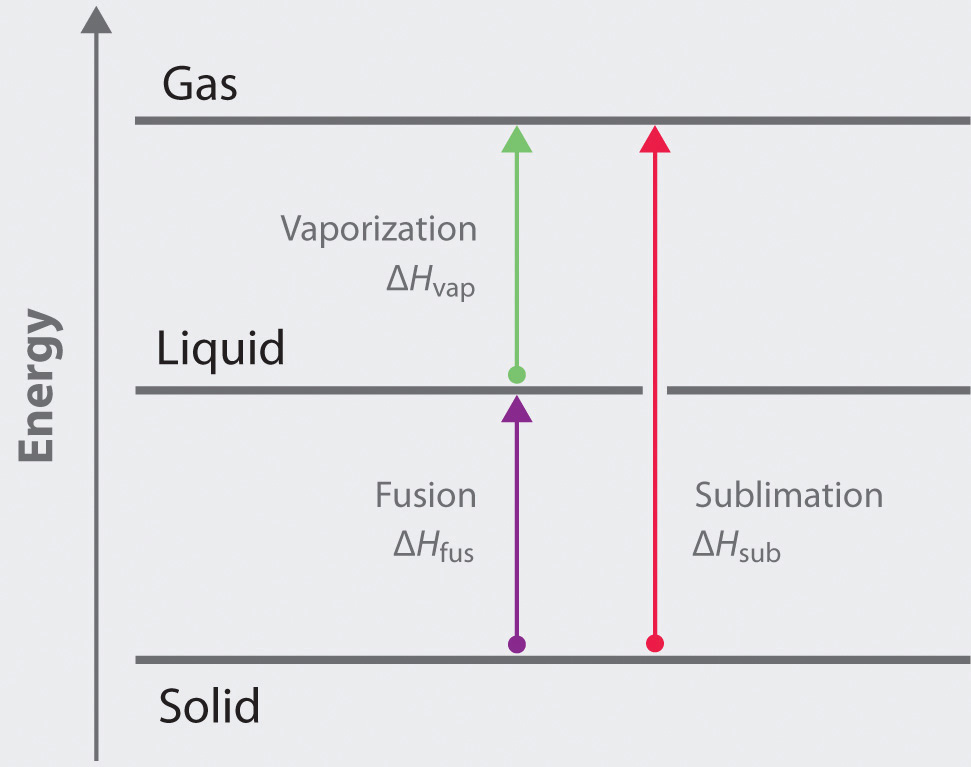 11 E Liquids And Intermolecular Forces Exercises Chemistry
11 E Liquids And Intermolecular Forces Exercises Chemistry
 The Structure Of Glass A Phase Equilibrium Diagram Approach
The Structure Of Glass A Phase Equilibrium Diagram Approach
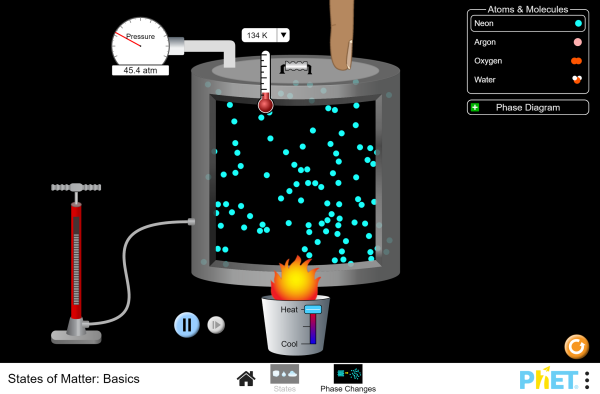 States Of Matter Basics Atoms Molecules States Of Matter
States Of Matter Basics Atoms Molecules States Of Matter
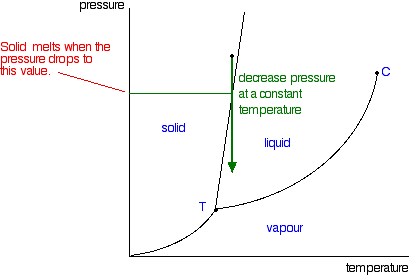 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
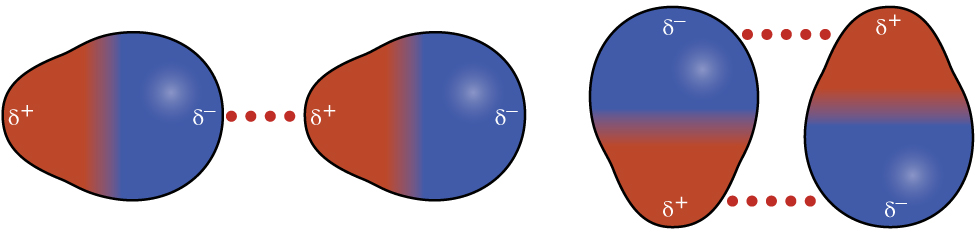 10 1 Intermolecular Forces Chemistry
10 1 Intermolecular Forces Chemistry
Argon Chemical Element Reaction Uses Elements Metal Gas
 The Structure Of Glass A Phase Equilibrium Diagram Approach
The Structure Of Glass A Phase Equilibrium Diagram Approach
 Solid Liquid Equilibrium An Overview Sciencedirect Topics
Solid Liquid Equilibrium An Overview Sciencedirect Topics
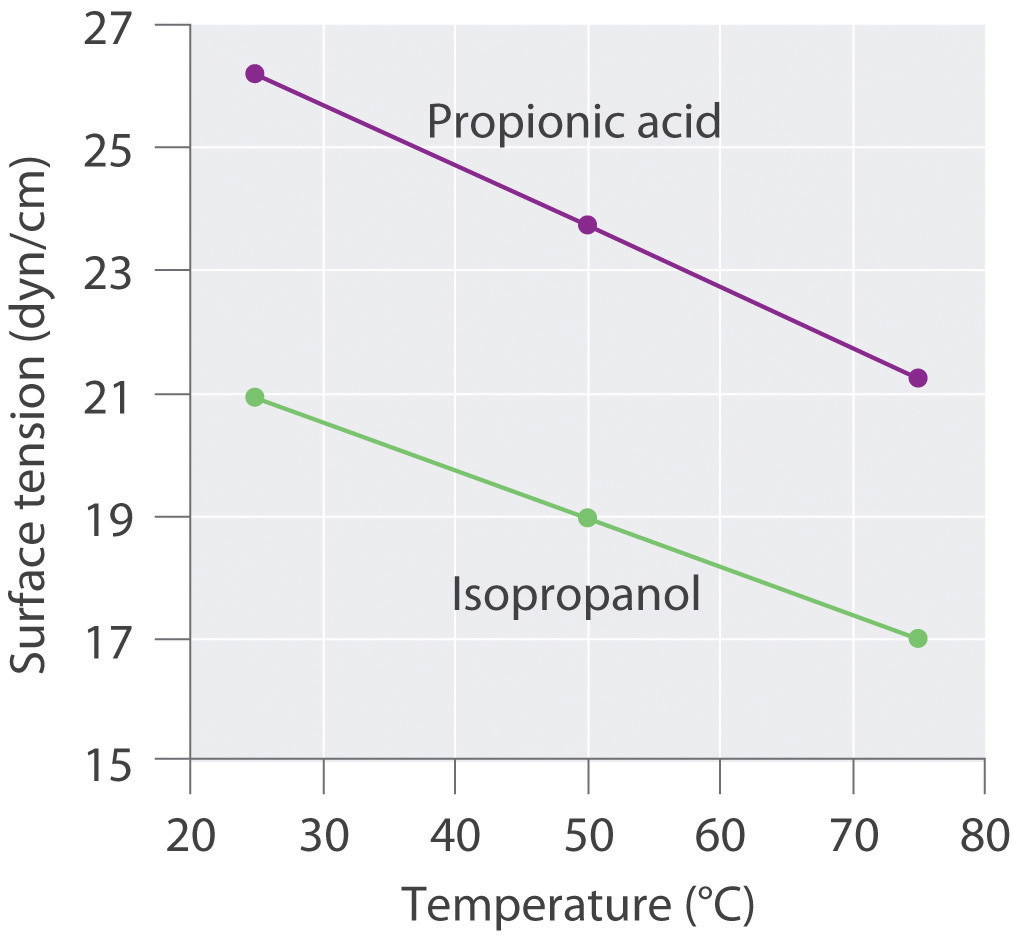 11 E Liquids And Intermolecular Forces Exercises Chemistry
11 E Liquids And Intermolecular Forces Exercises Chemistry
Properties Of Pure Substances Pure Substance Phases Of A Pure
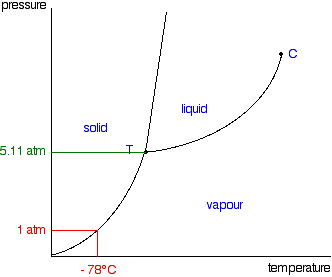 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
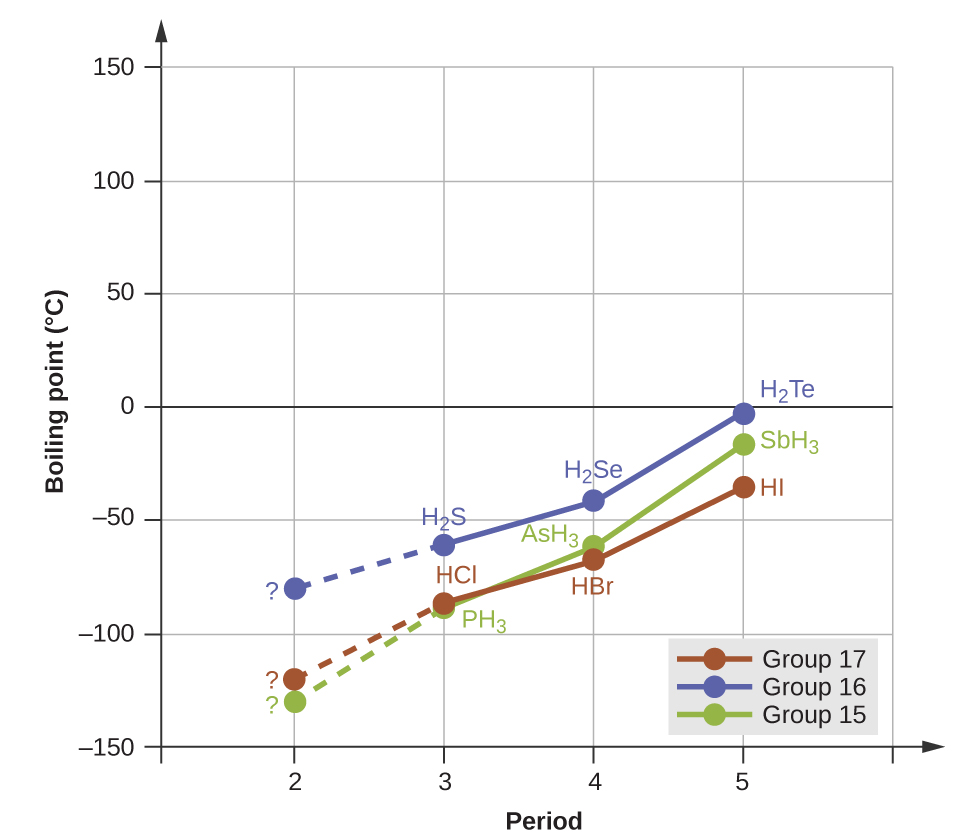 10 1 Intermolecular Forces Chemistry
10 1 Intermolecular Forces Chemistry
Fluid Phases Of Argon A Debate On The Absence Of Van Der Waals
Heat Energy And The States Of Matter
 10 1 Intermolecular Forces Chemistry
10 1 Intermolecular Forces Chemistry
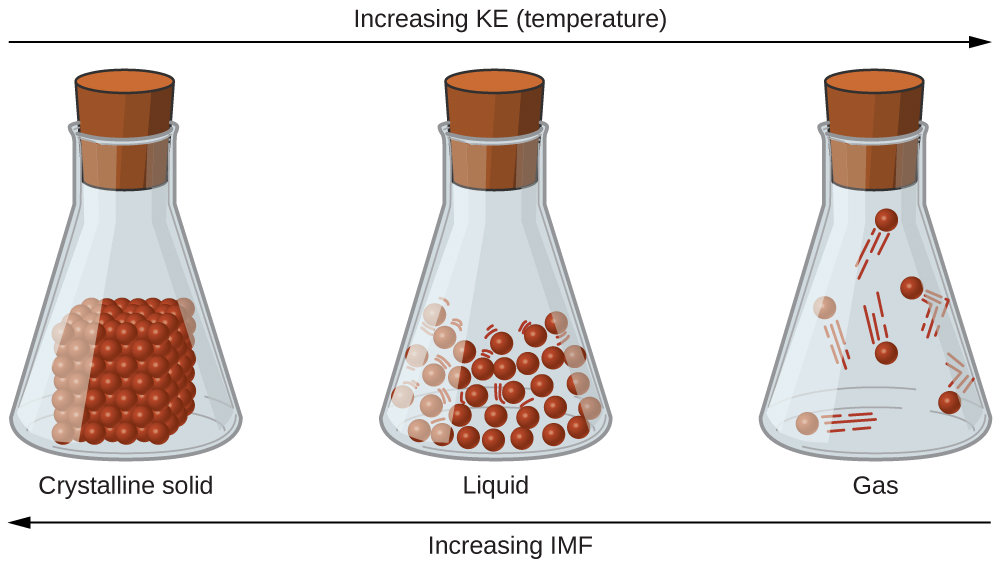 10 1 Intermolecular Forces Chemistry
10 1 Intermolecular Forces Chemistry
0 Response to "Sketch The Phase Diagram To Answer Whether Solid Argon Or Liquid Argon Has The Greater Density"
Post a Comment