Examine The Following Phase Diagram And Determine What Phase Exists At Point D
Calculuate the temperature at which its vapor pressure is exactly half of that at its normal boiling point. A vapor liquid b vapor c liquid d solid e supercritical fluid 4.
 A Possible Four Phase Coexistence In A Single Component System
A Possible Four Phase Coexistence In A Single Component System
A bos has a lower density than bol.
Examine the following phase diagram and determine what phase exists at point d. A dispersion forces b dipole dipole interactions c dipole. C bo changes from a solid to a liquid as one follows the line from c to d. A vapor liquid.
Examine the following phase diagram and identify the feature represented by point a. Examine the following phase diagram and determine what phase exists at point favapor liquidb. Examine the following phase diagram and determine.
Examine the following phase diagram and identify the feature represented by point a. Liquiddsolidesupercritical fluid 3 8. Chapter 12 consider the following phase diagram and identify the process occurring as one goes from point c to point d.
B london dispersion forces. Examine the phase diagram for the substance bogusium bo and select the correct statement. B the triple point for bo is at a higher temperature than the melting point for bo.
See question 10 image a bos has a lower density than bol. Sign up to view the full version. White fall 2013 10.
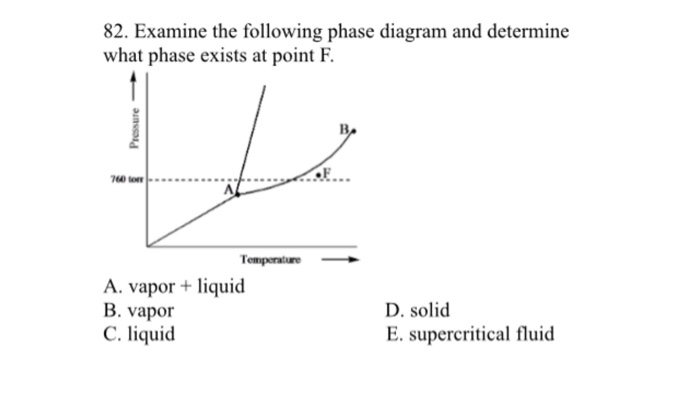
Increasing temperature with a phase change from solid to vapor examine the following phase diagram and determine what phase exists at point f. The heat of vaporization for either is 2669 kjmol. Examine the following phase diagram and identify the feature represented by point a and point b.
Point b in this phase diagram represents the only combination of temperature and pressure at which a pure substance can exist simultaneously as a solid a liquid and a gas. This preview has intentionally blurred sections. Ammonias unusually high melting point is the result of adipole dipole forces.
Examine the phase diagram for the substance bogusium bo and select the correct statement. Neon condenses due to a dipole dipole forces. B the triple point for bo is at a higher temperature than the melting point for bo.
Amelting point bcritical point ctriple point dsublimation point eboiling point 9. It is therefore called the triple point of the substance and it represents the only point in the phase diagram in which all three states are in equilibrium. Examine the following phase diagram and determine what phase exists at point f.
C bo changes from a solid to a liquid as one follows the line from c to d. Examine the phase diagram for the substance bogusium bo and select the correct statement. Examine the following phase diagram and determine what phase exists at point f.
Vapor which of the following intermolecular forces is the weakest. The normal boiling point of ether is 3078 k.
 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
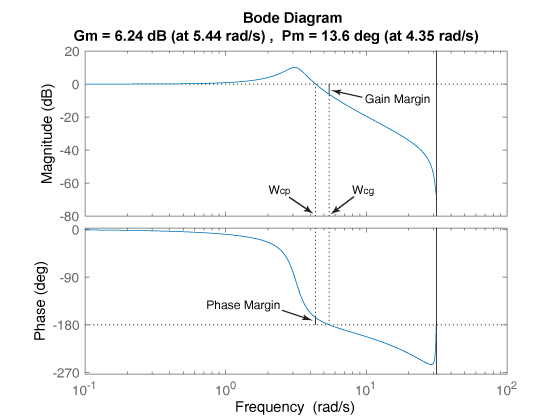 Gain Margin Phase Margin And Crossover Frequencies Matlab Margin
Gain Margin Phase Margin And Crossover Frequencies Matlab Margin
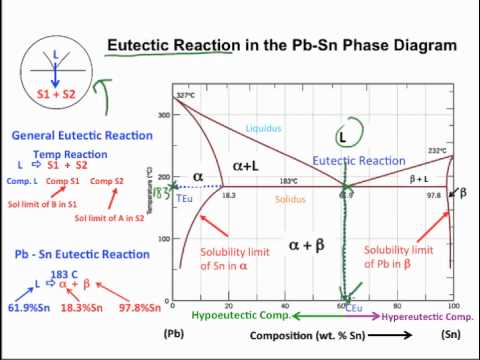 Muddiest Point Phase Diagrams I Eutectic Calculations And Lever
Muddiest Point Phase Diagrams I Eutectic Calculations And Lever
 Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
 Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
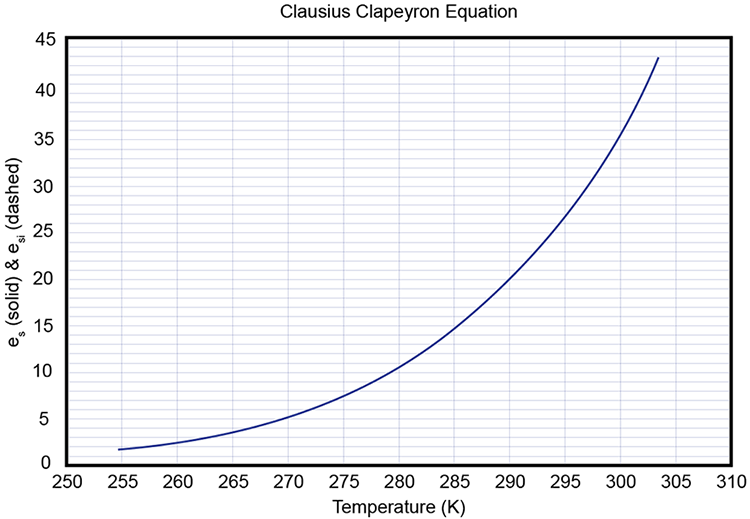 3 3 Phase Diagram For Water Vapor Clausius Clapeyron Equation
3 3 Phase Diagram For Water Vapor Clausius Clapeyron Equation
 Examine The Following Phase Diagram And Determine What Phase Exists
Examine The Following Phase Diagram And Determine What Phase Exists
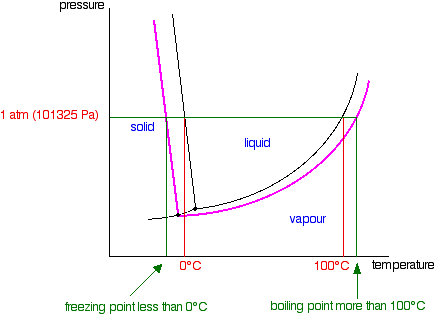 Raoult S Law And Non Volatile Solutes
Raoult S Law And Non Volatile Solutes
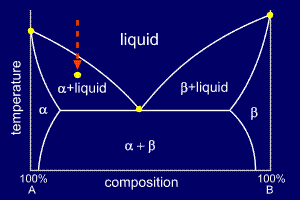
 A Possible Four Phase Coexistence In A Single Component System
A Possible Four Phase Coexistence In A Single Component System
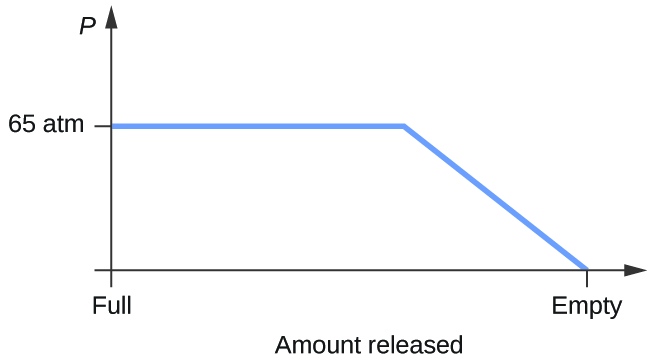

0 Response to "Examine The Following Phase Diagram And Determine What Phase Exists At Point D"
Post a Comment