Consider The Following Reaction Energy Diagram
Yes because energy is released consider the energy diagram for a chemical reaction in figure 6 3. How many elementary steps are in the reaction.
Which of the following is true about the potential energy diagram for 2h2 o2 2 h2o h 4866 kj.
Consider the following reaction energy diagram. Choose all that apply 1 the reaction is exothermic 2 the reacting molecules must collide with the correct orientation 3 heat is released 4 the reactants have a higher potential energy than the products 5 the reactants have a lower potential energy than the products 6 activation energy must. Consider the following reaction energy diagram. ½ h 2g ½ i 2g hi g h 28 kj.
Draw a rough sketch of the energy profile for each. The activation energy for the formation of hi is 167 kj. Home study science chemistry chemistry questions and answers consider the following reaction energy diagram.
Reaction a is exothermic and reaction b is endothermic. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Consider the following reaction.
For the following reaction profile indicatea. The activation energy for the following reaction is 125 kjmol and δe for the reaction is 216 kjmol. The activation energy in the diagram is 438 kcal mole letter c.
This state is also known as an activated complex. From the diagram when the temperature of reactants are raised from 20c to 30c. How many elementary steps are in the reaction me.
You have to note that activation energy is the energy needed for the reaction to occur and produce products. At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products. Consider two gases a and b that are in a container at room temperature.
The number of molecules having e r or greater doubles. Consider the following energy diagram showing the. Reaction a in endothermic and reaction b is exothermic.
The activation energy for the following reaction is 125 kjmol and δe for the reaction is 216 kjmol. Therefore the spike after h2 and i2 is reacted is the activation energy of the reaction. Does this provide evidence that a chemical reaction took place.
Ch 10 Kinetic And Thermodynamic Control
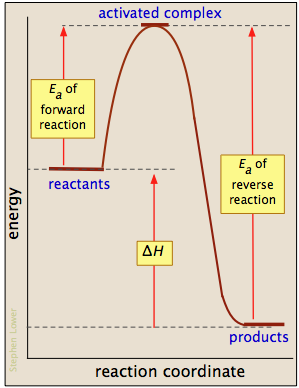 Basics Of Reaction Profiles Chemistry Libretexts
Basics Of Reaction Profiles Chemistry Libretexts
 Reaction Energy Diagram Of The Dehydrogenation Reactions Of Ammonia
Reaction Energy Diagram Of The Dehydrogenation Reactions Of Ammonia
Catalysts In 21st Century Energy
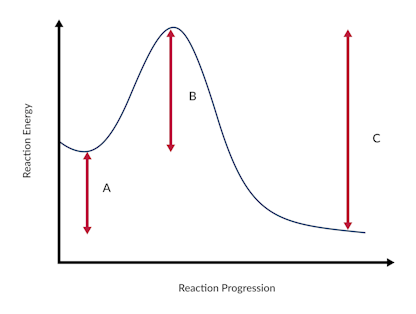 Ngss Physical Sciences Reaction Energy Diagram
Ngss Physical Sciences Reaction Energy Diagram
 How Would You Draw And Label Energy Diagrams That Depict The
How Would You Draw And Label Energy Diagrams That Depict The
What Is The Activation Energy For A Reverse Reaction Quora

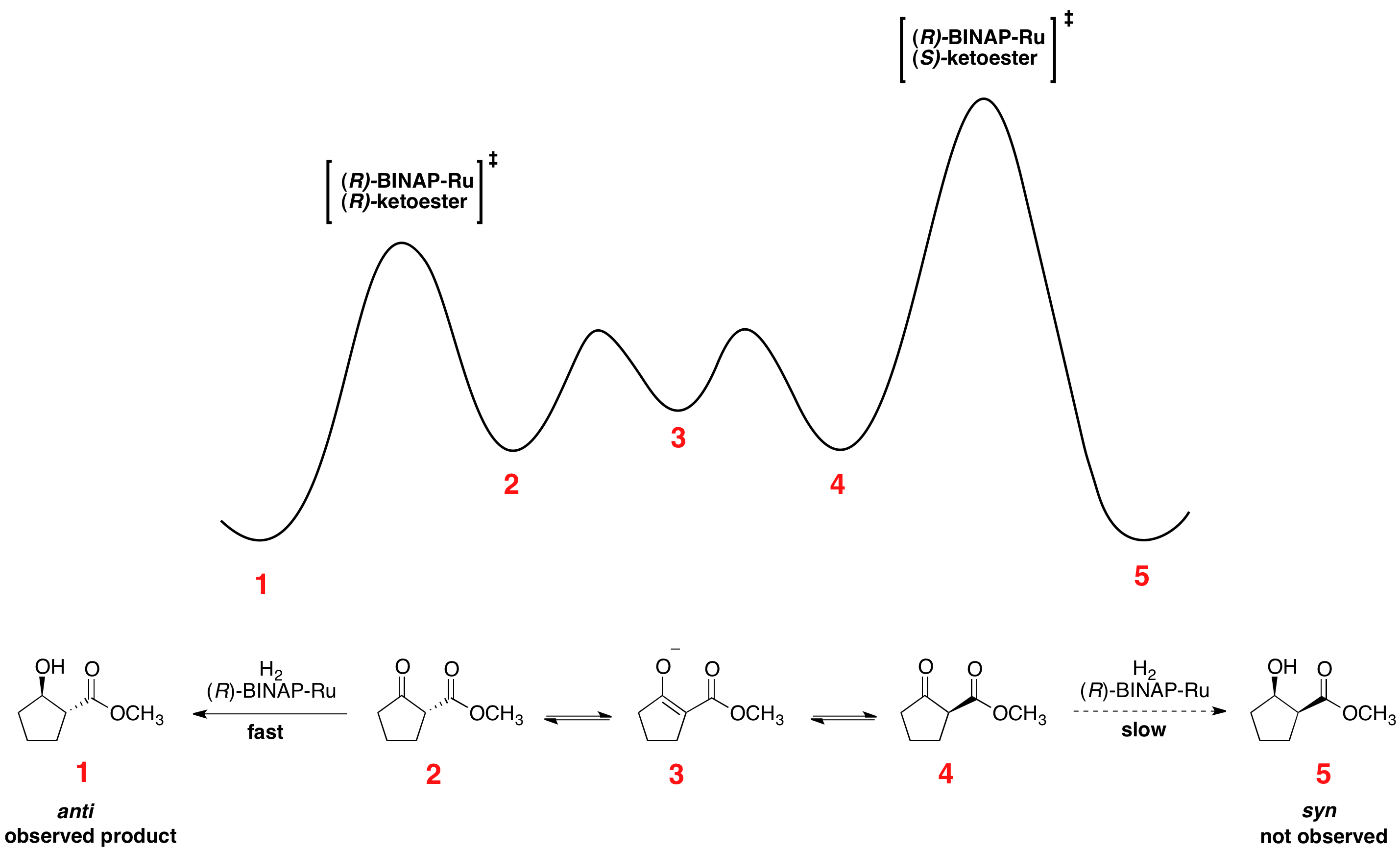 File Energy Diagram For The Noyori Asymmetric Hydrogenation Png
File Energy Diagram For The Noyori Asymmetric Hydrogenation Png
Illustrated Glossary Of Organic Chemistry Rate Determing Step
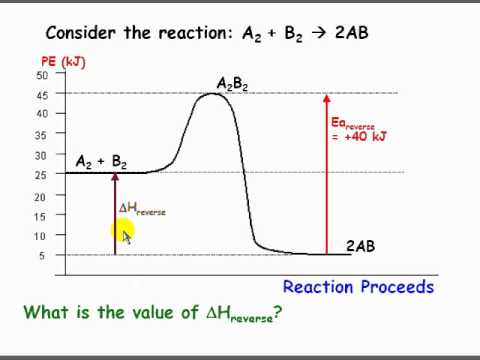 Using Potential Energy Diagrams Flv Youtube
Using Potential Energy Diagrams Flv Youtube
 Answer Consider The Following Reaction Clutch Prep
Answer Consider The Following Reaction Clutch Prep
 Thermodynamics And Reactive Intermediates Energy Diagrams Youtube
Thermodynamics And Reactive Intermediates Energy Diagrams Youtube
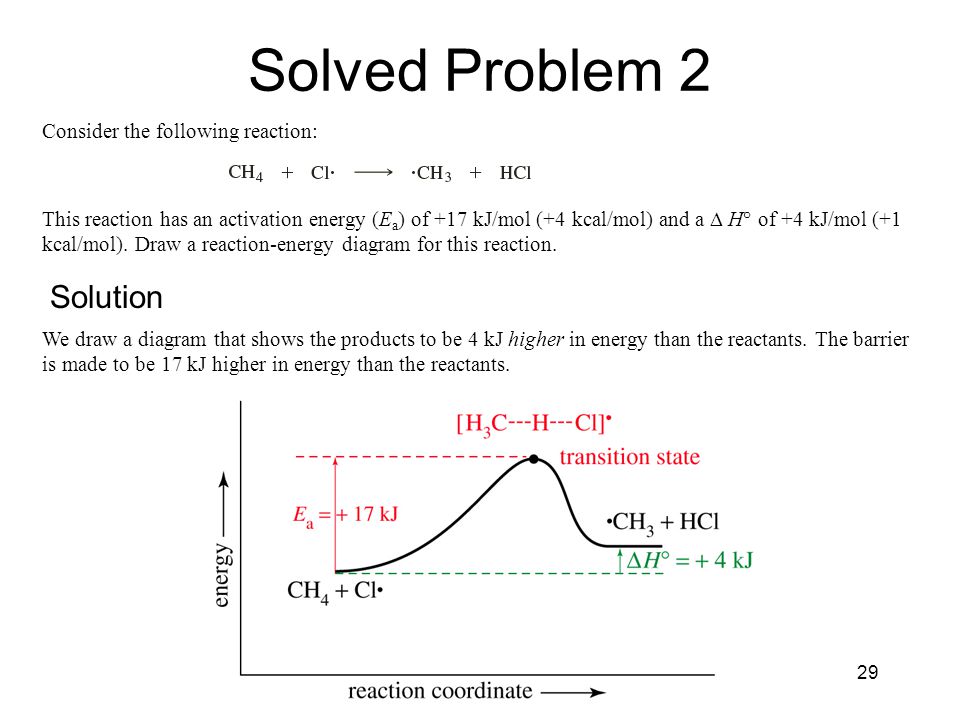 The Study Of Chemical Reactions Ppt Video Online Download
The Study Of Chemical Reactions Ppt Video Online Download
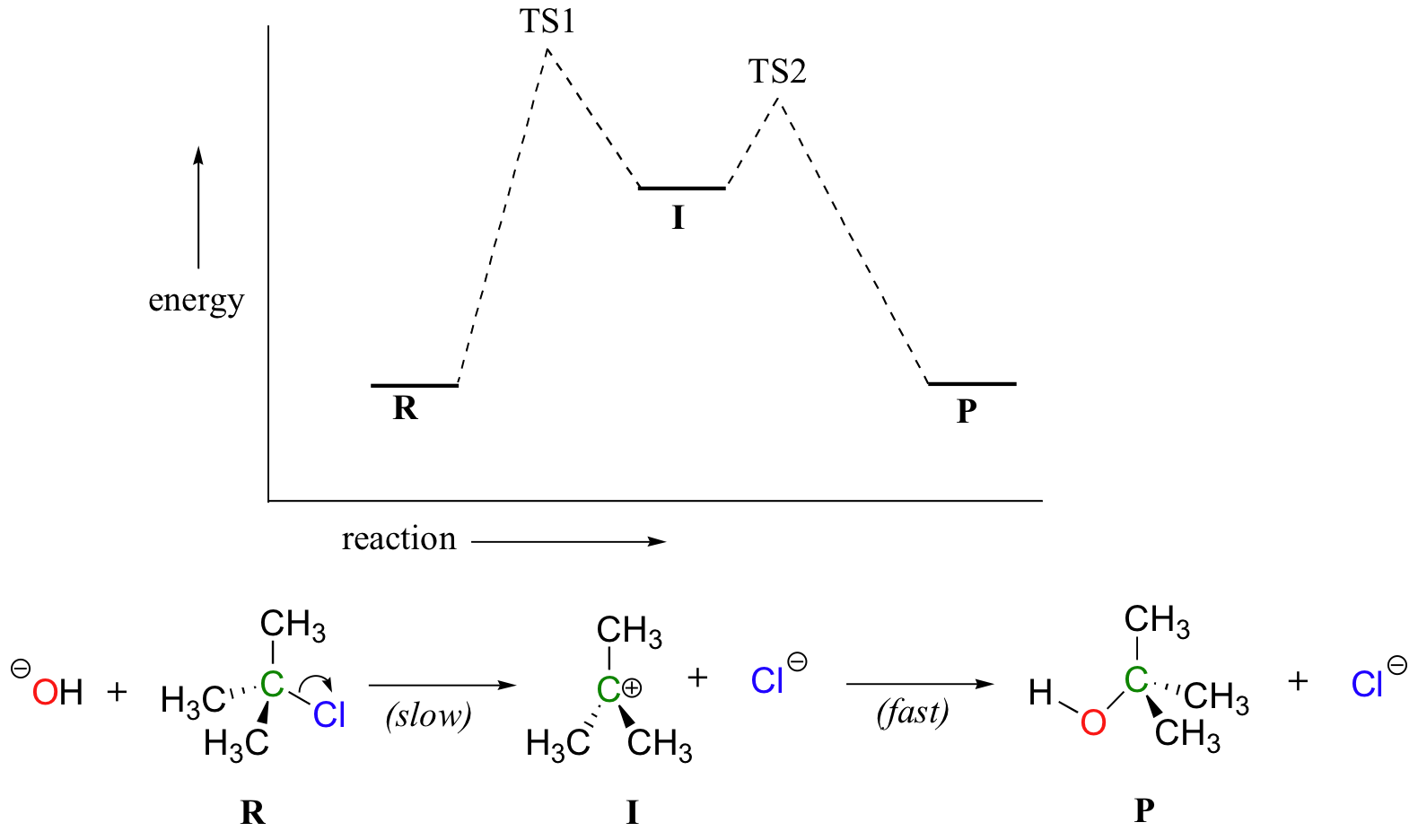 6 2 Energy Diagrams Chemistry Libretexts
6 2 Energy Diagrams Chemistry Libretexts
 Mechanisms And Potential Energy Diagrams Chemistry For Non Majors
Mechanisms And Potential Energy Diagrams Chemistry For Non Majors
 Solved Look At The Following Energy Diagram A Is Dg For The
Solved Look At The Following Energy Diagram A Is Dg For The
 Reaction Coordinate Diagrams College Chemistry
Reaction Coordinate Diagrams College Chemistry
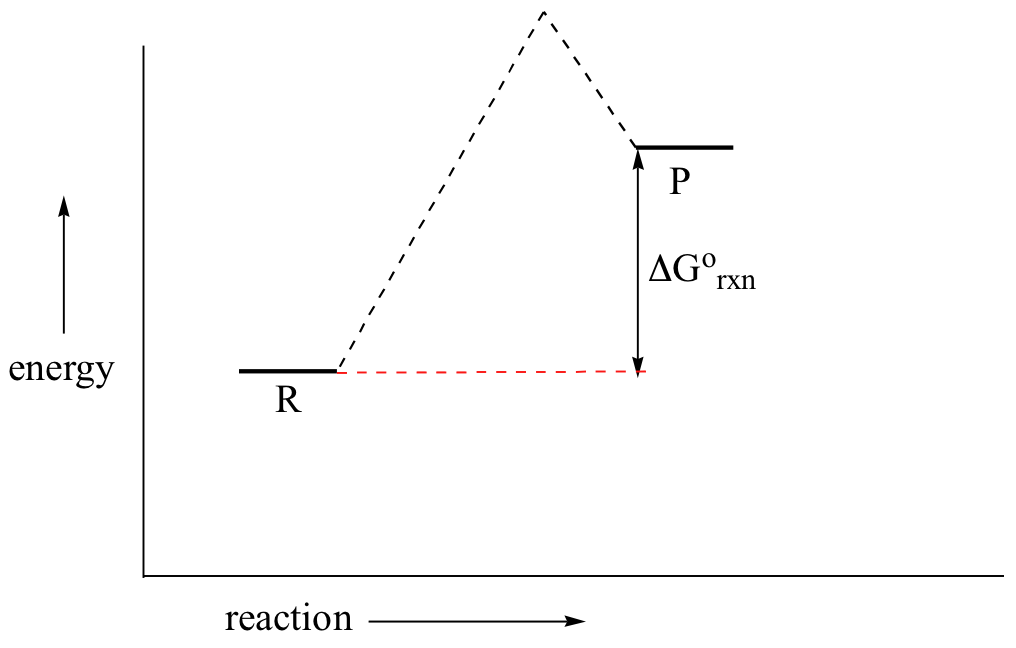 6 2 Energy Diagrams Chemistry Libretexts
6 2 Energy Diagrams Chemistry Libretexts
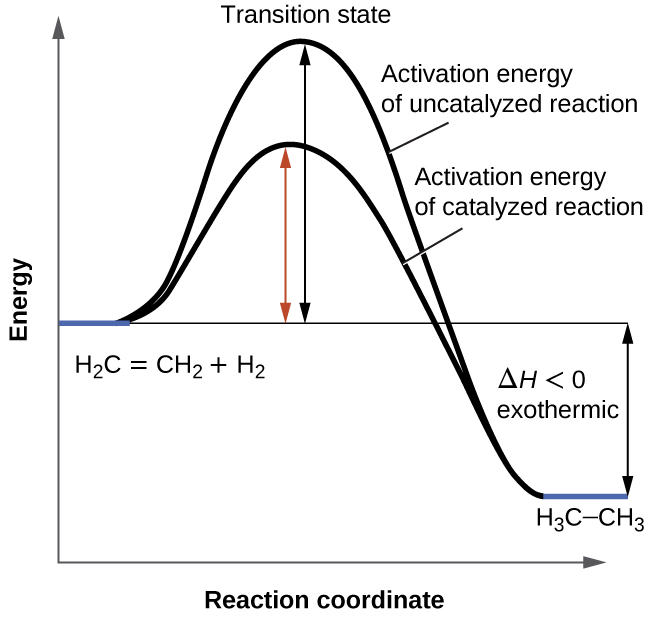
0 Response to "Consider The Following Reaction Energy Diagram"
Post a Comment