Construct The Orbital Diagram Of Each Atom Or Ion
Electron configuration of the fluoride ion f. Write orbital diagram for the followingsa.
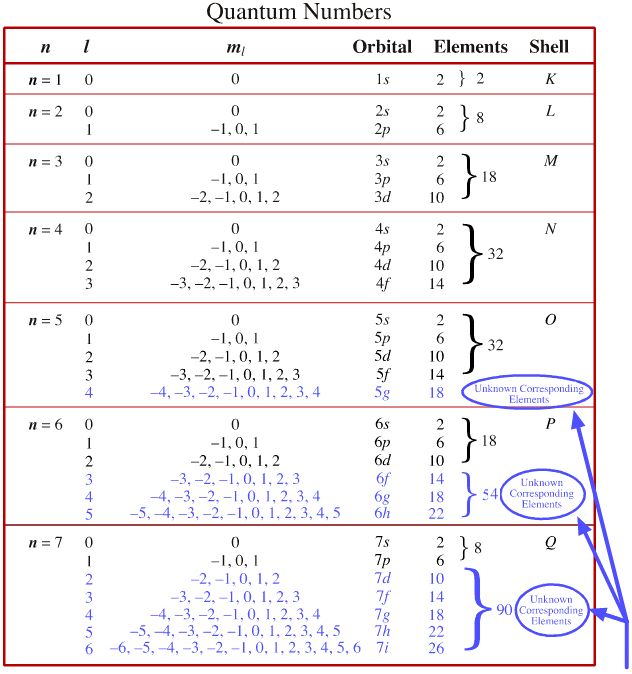
 11 5 Molecular Orbital Theory Chemistry Libretexts
11 5 Molecular Orbital Theory Chemistry Libretexts
So the answer is 7.
Construct the orbital diagram of each atom or ion. Previous question next question. Construct the orbital diagram of each atom or ion. If you took two electrons away due to the 2 charge you would have 4s13d1.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1 core notation. The electron configuration for an element is the arrangement of electrons in the orbits or shells of a neutral atom. How many electrons does a fe atom have in its 3.
Construct the orbital diagram of each atom or ion. Show transcribed image text construct the orbital diagram of each atom or ion. Construct the orbital diagram of each atom or ion.
Its maximum capacity is actually 6 electrons two electrons for each p orbital. Draw the orbital diagram for ion co 2. Construct the orbital diagram of each atom or iontiti2ti4 problem.
Scandium 1s 2s 2p 3s 3p 4s 3d full electron configuration. Normally you would expect the electron configuration of ti to be ar 4s23d2 but in actuality it is 4s13d3. Now the f anion is formed when 1 electron is added to a neutral fluorine atom.
Notice that the 2p subshell of the neutral atom contains 5 electrons. Neutral fluorine has 9 protons and 9 electrons in its orbitals but the fluoride ion has an extra electron giving it a total of 10 electrons and their configuration is 1s2 2s2 2p6 the same as that of a neon atom. Ti ti2 ti4 construct the orbital diagram of each atom or ion.
Construct the orbital diagram of each atom or ion. Draw the orbital diagram for the valence shell of. Ti 2 ti 4 next.
Choose the orbital diagram that represents the gro. Answer to construct the orbital diagram of each atom or ion. Draw the orbital diagram for ion ca 2.
Ar 4s 2 3d 1 2. This means that the extra electron will be added to one of the three 2p orbitals. Consider the portion of the orbital filling diagra.
Draw orbital diagrams for the following elements. Shells closer to the nucleus have higher binding energy. Construct the orbital diagram of each atom or ion.
Choice b is the best answer here. So if you count the electrons of the ti2 in the s orbital only you have 1 from each of h he li be na mg and k. For example there are eleven electrons in a sodium atom atomic number 11.
Get more help from chegg. Write the electron configuration full and in core notation. 85 26 ratings this problem has been solved.
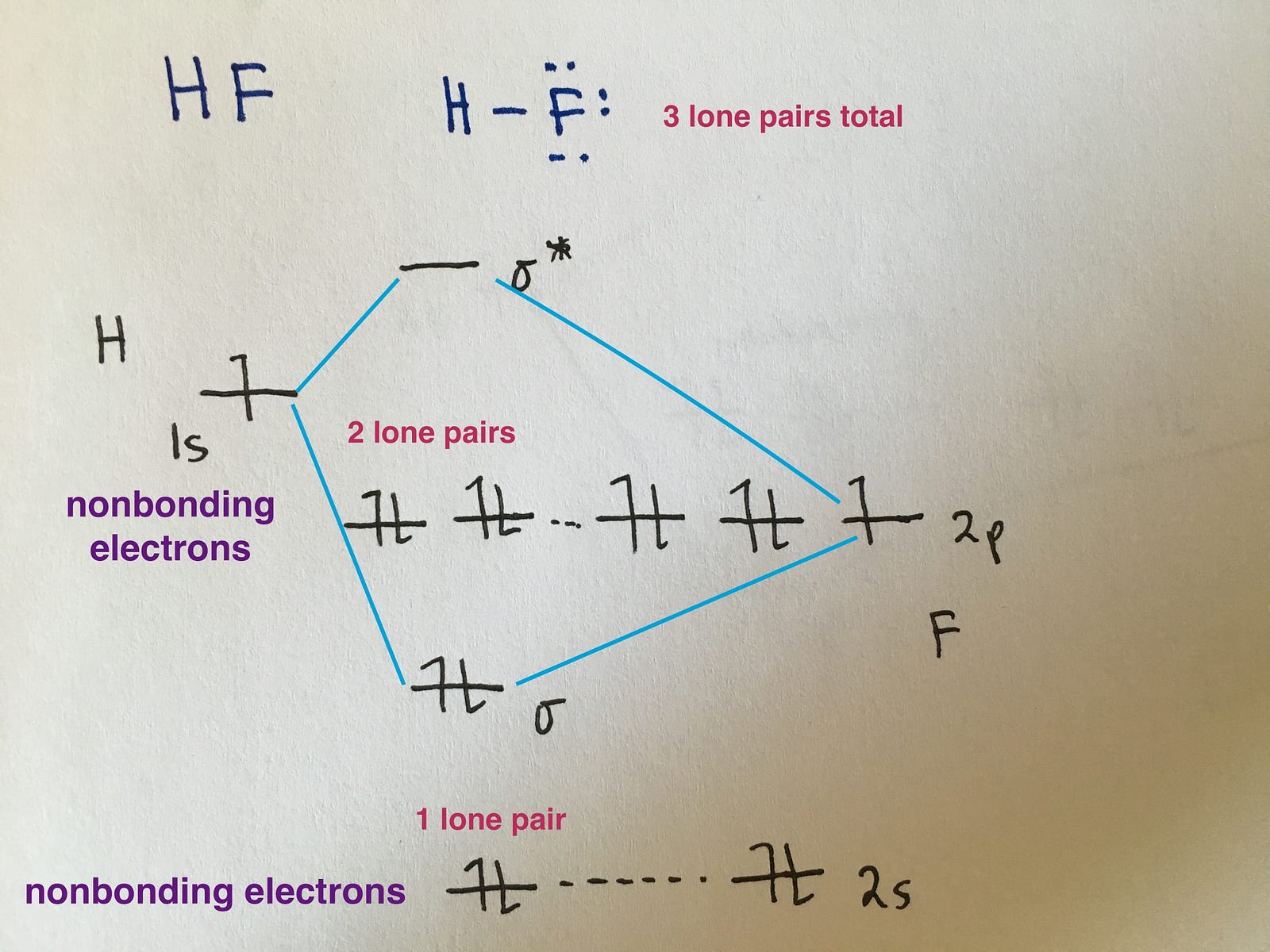 Molecular Orbital Diagrams Simplified Megan Lim Medium
Molecular Orbital Diagrams Simplified Megan Lim Medium
 Quantum Number Periodic Table Chemogenesis
Quantum Number Periodic Table Chemogenesis
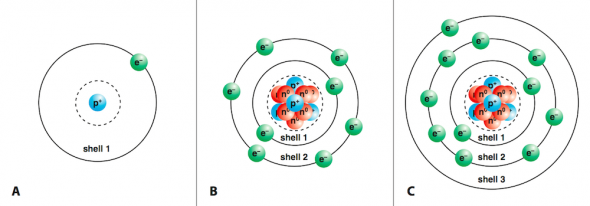 Ionic Compounds Manoa Hawaii Edu Exploringourfluidearth
Ionic Compounds Manoa Hawaii Edu Exploringourfluidearth
Ch105 Chapter 3 Ionic And Covelent Bonding Chemistry
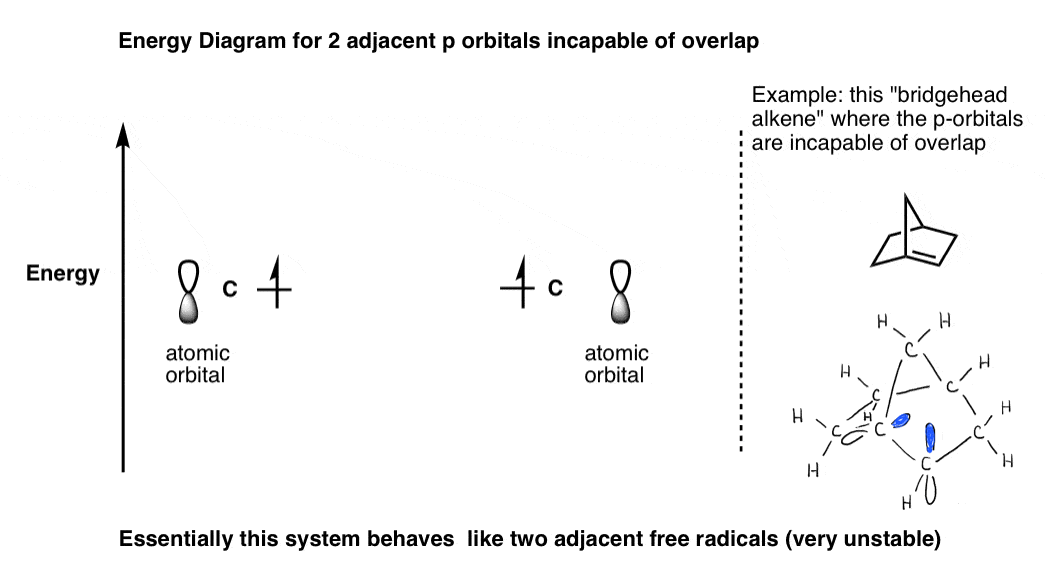 Molecular Orbital Diagram For A Simple Pi Bond Bonding And
Molecular Orbital Diagram For A Simple Pi Bond Bonding And
Polyatomic Molecular Orbital Theory
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
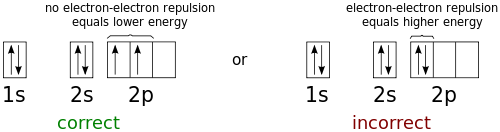 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
 What Is The Electron Configuration Of F Socratic
What Is The Electron Configuration Of F Socratic
Answers To Self Tests And Exercises
 Electron Configurations Html Chm2045 F13 All
Electron Configurations Html Chm2045 F13 All
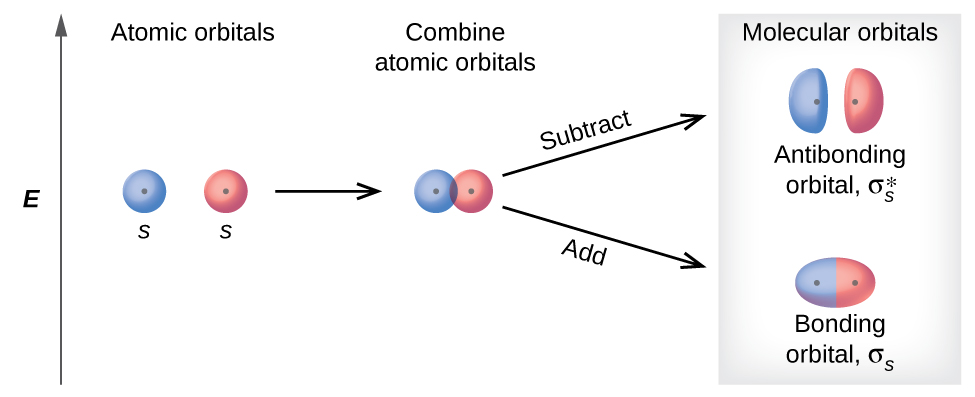 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
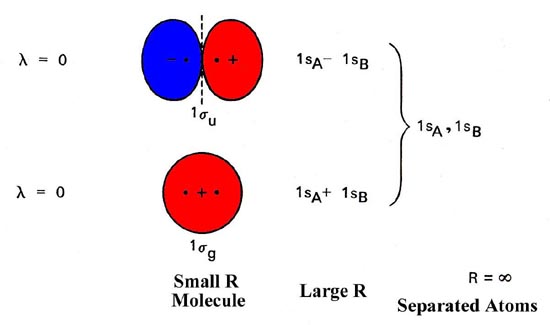 Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
Bonding Orbitals In Ammonia Sp3 Hybrids
 Molecular Orbital Diagram For A Simple Pi Bond Bonding And
Molecular Orbital Diagram For A Simple Pi Bond Bonding And
0 Response to "Construct The Orbital Diagram Of Each Atom Or Ion"
Post a Comment