Use The Following Mo Diagram To Find The Bond Order For O2
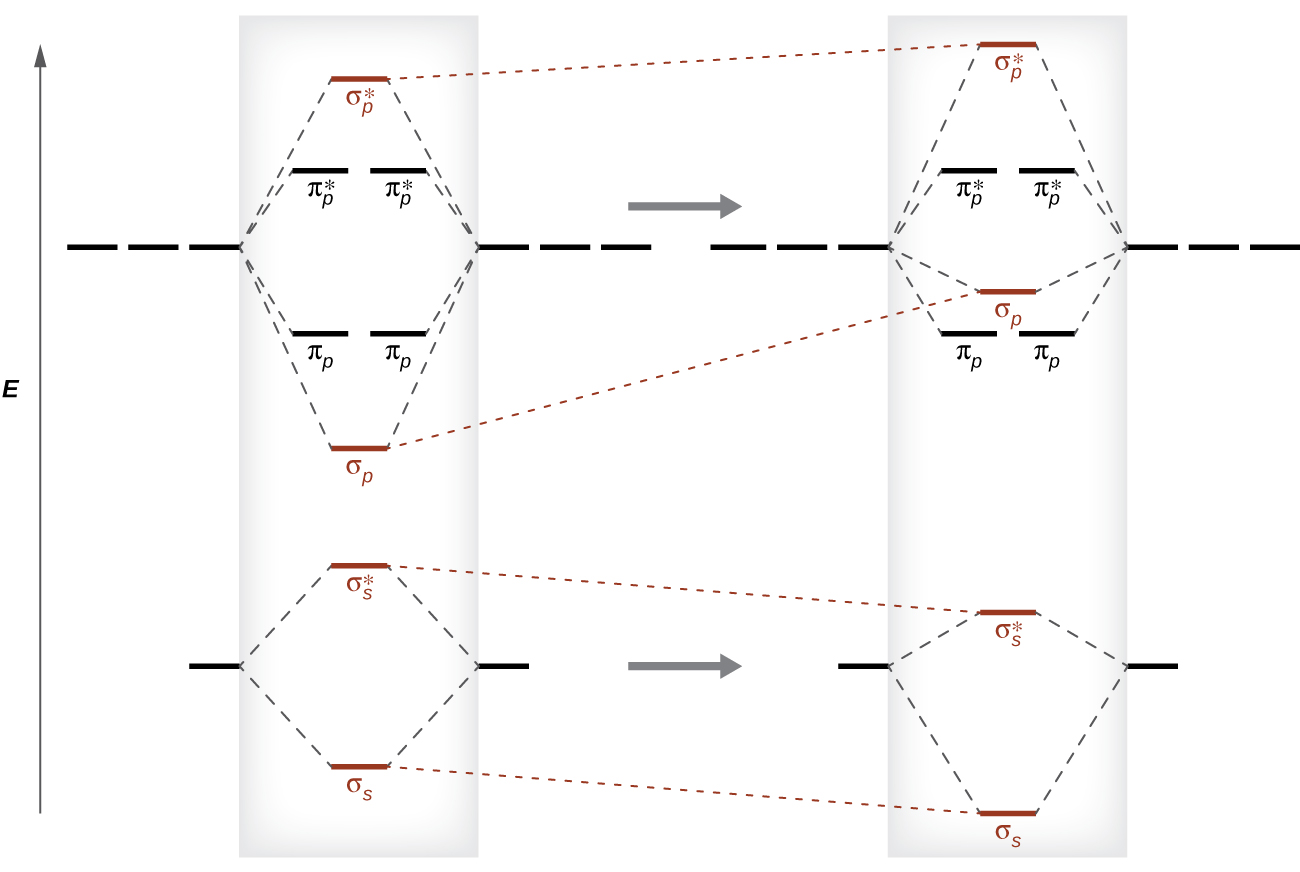
The relative energies of the sigma orbitals drop below that of the pi orbitals. A write the molecular orbital diagram as in example.
 4 Sketch The Molecular Orbital Diagrams With Ele Chegg Com
4 Sketch The Molecular Orbital Diagrams With Ele Chegg Com
Use a molecular orbital diagram to determine the bond order in the o 2 ion.
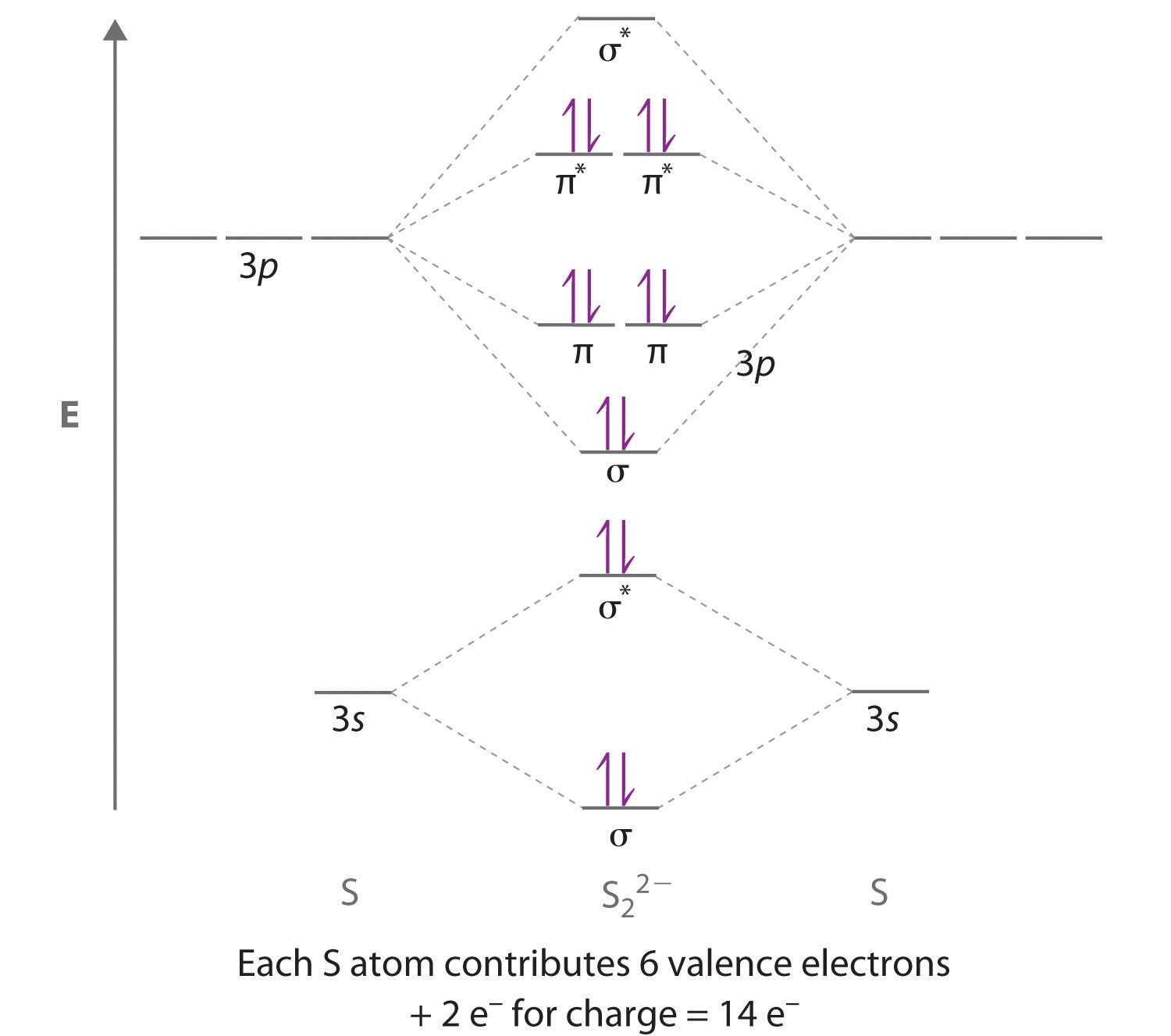
Use the following mo diagram to find the bond order for o2. Calculate bond order for simple molecules. C determine if the species is diamagnetic or paramagnetic. Draw the lewis structure for the following molecules.
Molecular orbital diagram for oxygen gas o2. The bond order in sulfur dioxide for example is 15 the average of an s o single bond in one lewis structure and an so double bond in the other. Determine the bond order in each of these ions.
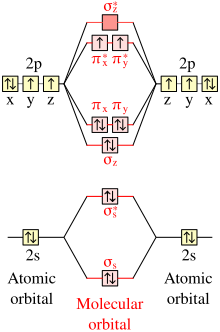
And if paramagnetic indicate the number of unpaired electrons. In its most basic form the bond order is the number of bonded electron pairs that hold two atoms together. Information from the mo diagram justify o2s stability and show that its bonding order is 2.
Molecular orbitals mo are constructed from atomic orbitals. Bonding order is 2 and it is paramagnetic. A triple covalent bond three and so on.
Construct a molecular orbital diagram and write the valence electron configuration of the dicarbon anion and cation c 2 and c 2. Consider how atoms come together into molecules. Determine the magnetism of simple molecules.
A double covalent bond a bond order of two. Write a valence electron configuration s 2s 2 for this ion. Example bdetermine the bond order and state whether you expect the species to be stable or unstable.
Fill in the mo diagram that corresponds to each of the molecules given. A single covalent bond has a bond order of one. In o 2 and f 2 there is a crossover of the sigma and the pi ortbials.
In molecular orbital theory we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital cancel the effect of one bond. Fill from the bottom up with 12 electrons total. Molecular orbital diagrams of diatomic molecules.
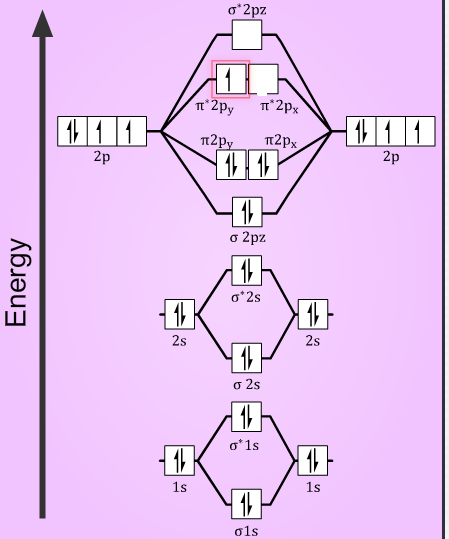 More Stable Among O2 And O2 Chemical Bonding And Molecular
More Stable Among O2 And O2 Chemical Bonding And Molecular
 By Writing Molecular Orbital Configuration For No Co O2 Molecules
By Writing Molecular Orbital Configuration For No Co O2 Molecules
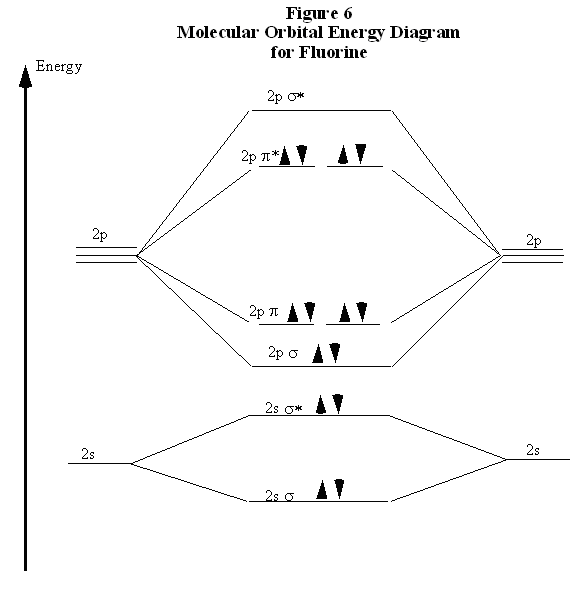 Ionization Energies Of Diatomic Molecule Chemistry Libretexts
Ionization Energies Of Diatomic Molecule Chemistry Libretexts
 Molecules Molecular Orbital Theory And No Of Bonds Chemistry
Molecules Molecular Orbital Theory And No Of Bonds Chemistry
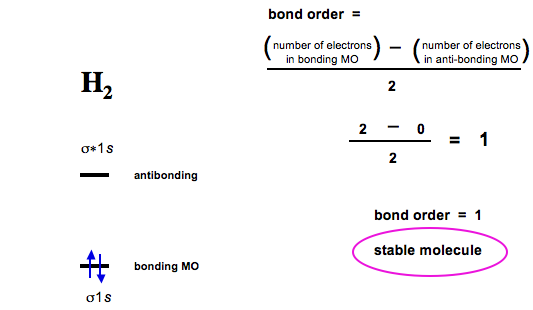 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 What Is The Bond Order Of Oxygen Quora
What Is The Bond Order Of Oxygen Quora
What Is An F2 Bond Order Quora
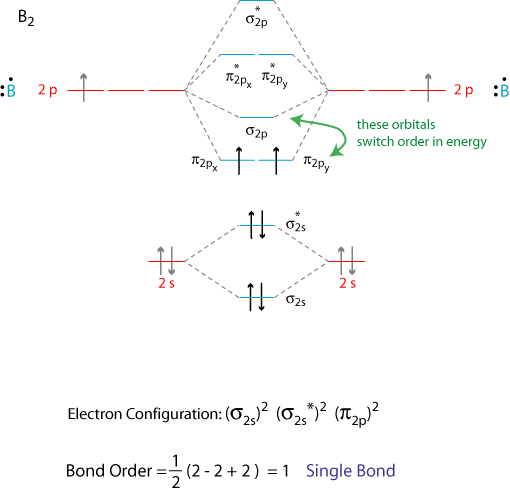 Molecular Orbital Theory Grandinetti Group
Molecular Orbital Theory Grandinetti Group
 Chapter 6 5 Delocalized Bonding And Molecular Orbitals Chemistry
Chapter 6 5 Delocalized Bonding And Molecular Orbitals Chemistry
 Solved Molecular Orbitals And Period 2 Diatomic Molecules Sec
Solved Molecular Orbitals And Period 2 Diatomic Molecules Sec
Introduction To Molecular Orbital Theory
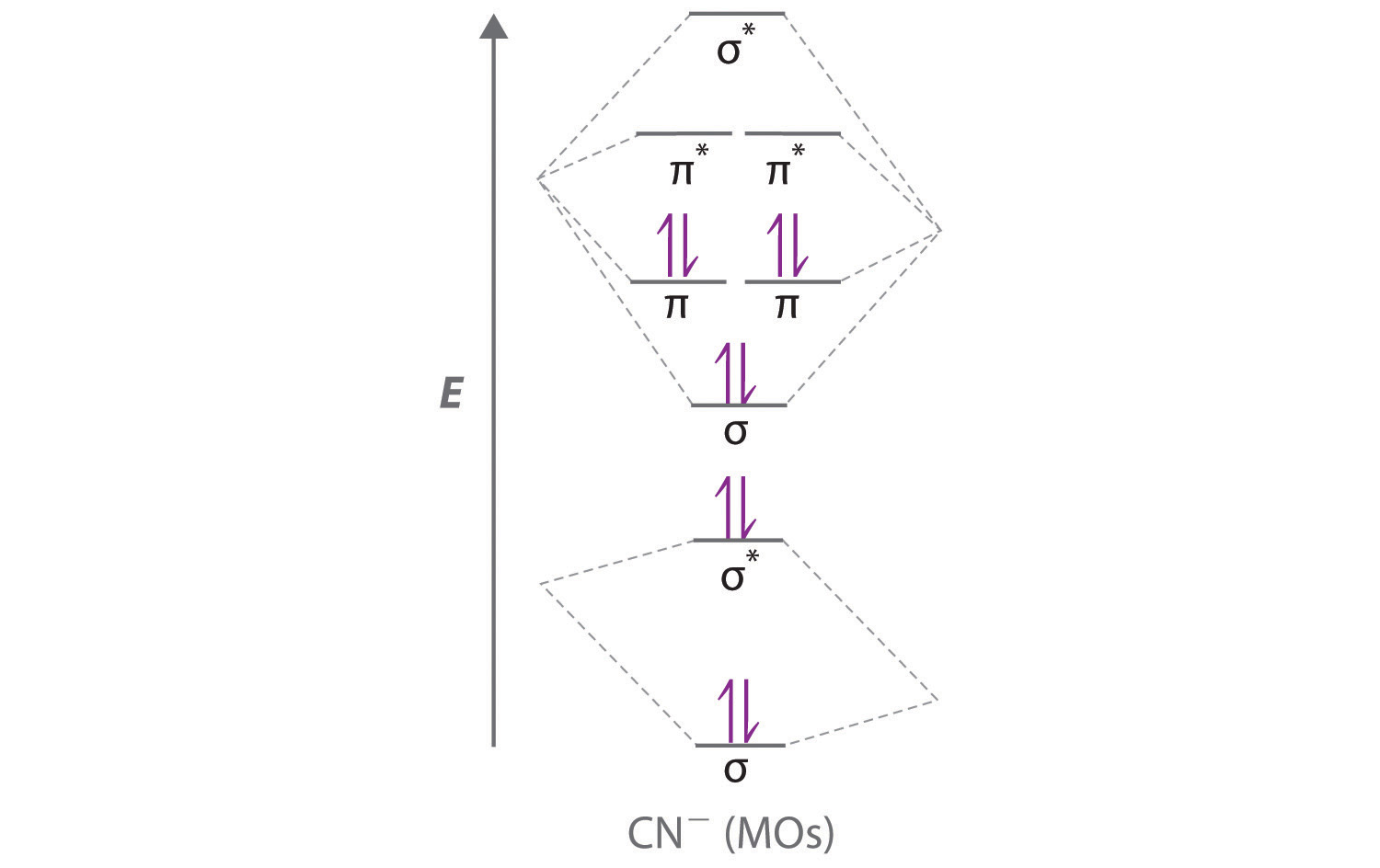
 Molecular Orbital Theory Heteronuclear Diatomic Cyanide Cn
Molecular Orbital Theory Heteronuclear Diatomic Cyanide Cn
Chemistry The Central Science Chapter 9 Section 8
 Molecular Orbital Theory Ppt Video Online Download
Molecular Orbital Theory Ppt Video Online Download
 Delocalized Bonding And Molecular Orbitals
Delocalized Bonding And Molecular Orbitals
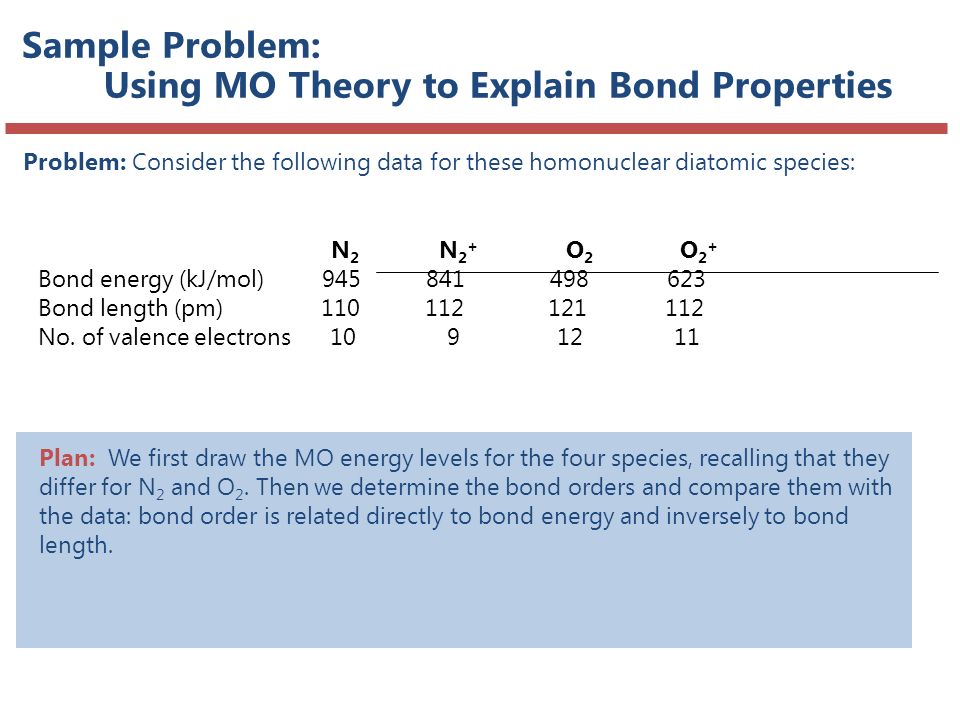 What Do Molecules Look Like Ppt Download
What Do Molecules Look Like Ppt Download
 Molecular Geometry And Bonding Theories Ppt Video Online Download
Molecular Geometry And Bonding Theories Ppt Video Online Download
 Constructing The O2 Molecular Orbital Energy Level Diagram Youtube
Constructing The O2 Molecular Orbital Energy Level Diagram Youtube
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
0 Response to "Use The Following Mo Diagram To Find The Bond Order For O2"
Post a Comment