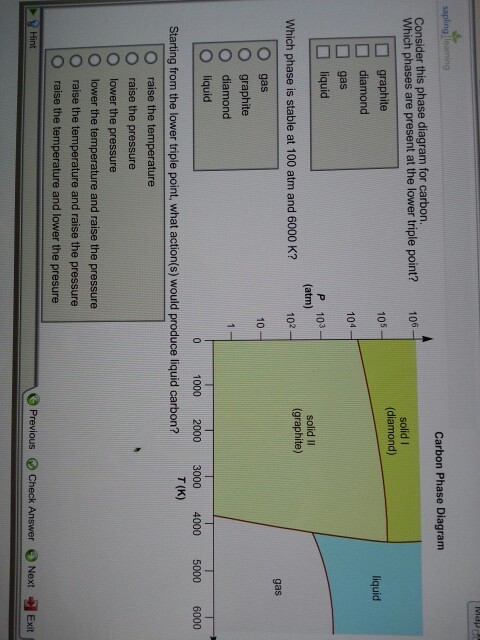
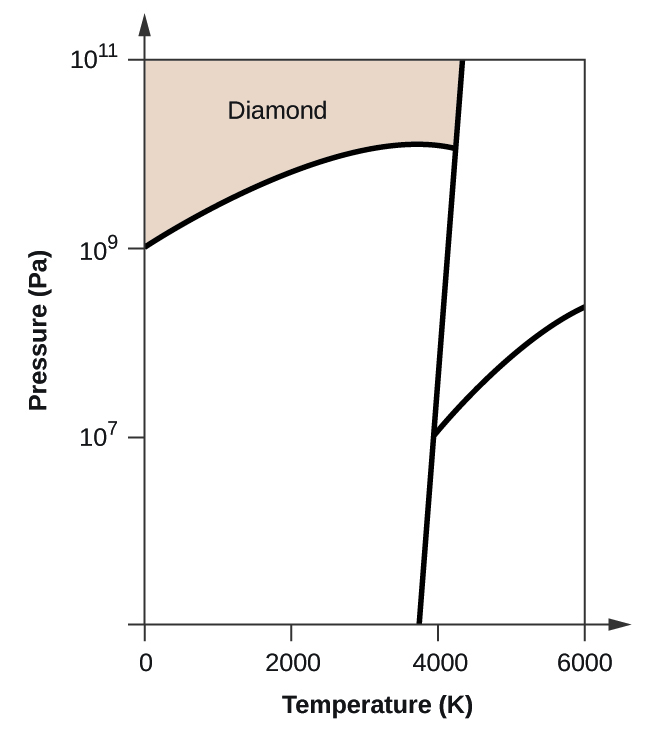
Consider This Phase Diagram For Carbon Which Phases Are Present At The Upper Triple Point
Knowing what the triple point means and where the triple point is. Which phases are present at the lower triple point.
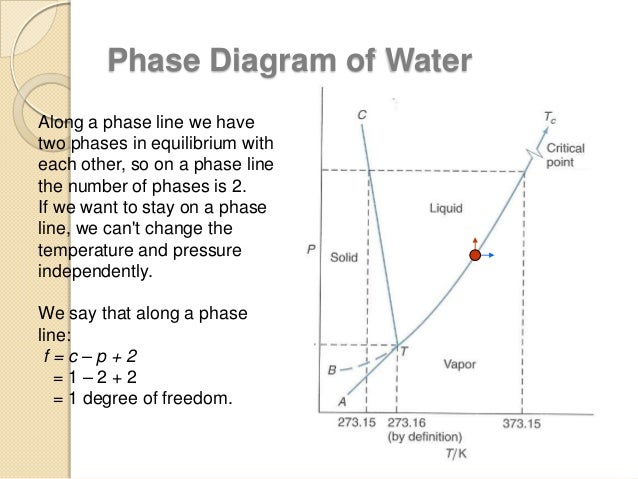
That is if one watches the container the amounts of vapor and liquid do not change.
Consider this phase diagram for carbon which phases are present at the upper triple point. Diamond graphite gas liquid which phase is stable at 105 atm and 1000 k. A substance can exist in equilibrium as a solid liquid and gas at this point. This is the point at which the phase boundary between liquid and gas terminates.
This is the reason that solid carbon dioxide is often known as dry ice. Consider the phase diagram for carbon dioxide shown in figure 5 as another example. 1 the substance cannot exist in the liquid form.
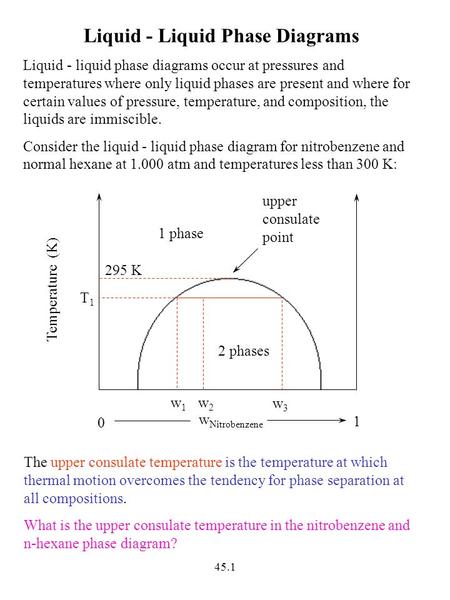
Notice that the triple point is well above 1 atm indicating that carbon dioxide cannot exist as a liquid under ambient pressure conditions. Raise the temperature raise the pressure lower the pressure lower the temperature and raise the pressure raise the temperature and lower the pressure raise the. The phase diagram for carbon dioxide.
Choose the statement below that is true. D circle each triple point on the phase diagram. Phase diagrams and the triple point.
Gas liquid diamond graphite starting from the lower triple point what actions would produce liquid carbon. A the triple point is where gas liquid and solid exist together. Use phase diagrams to identify stable phases at given temperatures and pressures and to describe phase transitions resulting from changes in these properties.
What phases are present at the triple point and the critical point. Problems and solutions book. Refer to the phase diagram for carbon dioxide in problem set 60.
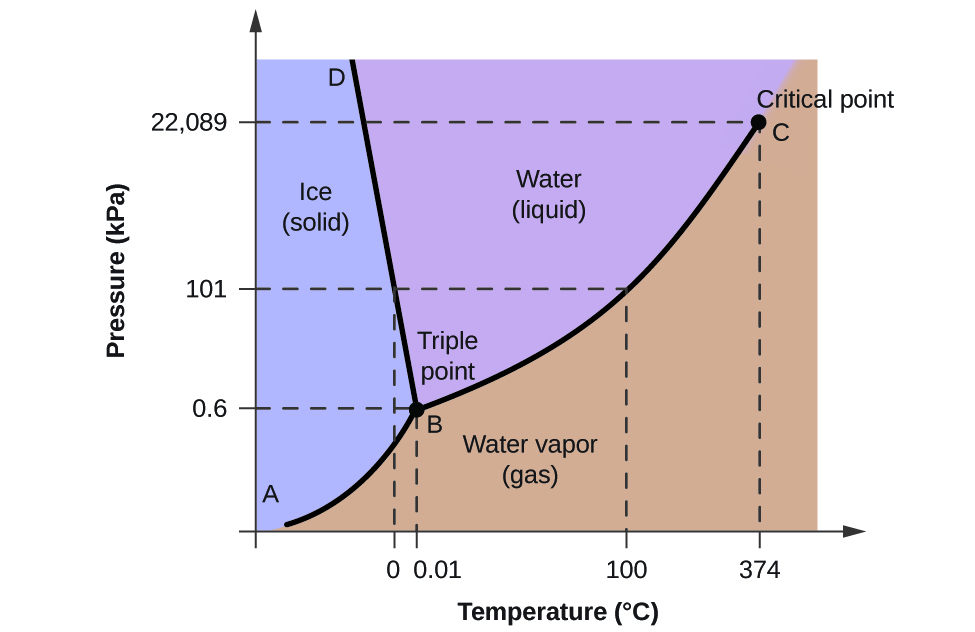
The vapor is in equilibrium with the liquid. The solid liquid curve exhibits a positive slope indicating that the melting point for co 2 increases with pressure as it does for most substances water being a notable exception as described previously. This container has only water in vapor and liquid formno air or any other substance.
Which of the following occurs when the temperature and pressure are below the triple point of a substance. C the solid phase of this substance is higher in density than the liquid phase. They tell you that you start with the liquid phase co2 l at 30 atm and 450k but b they tell you that it is being released to 1 atm and 298 k.
Phase diagrams in the chemistry. Consider the phase diagram shown. That means that at 1 atmosphere pressure carbon dioxide will sublime at a temperature of 78c.
B at 10 atm of pressure there is no temperature where the liquid phase of this substance would exist. Consider this phase diagram for carbon. Here are the facts.
Consider an isolated adiabatic container of water at 100 c. Label the diamond phase. Which phases are present at the lower triple point.
You cant get liquid carbon dioxide under normal conditions only the solid or the vapour. A the triple point of this substance occurs at a temperature of 31c. Consider this phase diagram for carbon.
Consider the phase diagram for carbon dioxide shown in figure 5 as another example.
 Experimental Pressure Temperature Phase Diagram Of Boron Resolving
Experimental Pressure Temperature Phase Diagram Of Boron Resolving
Towards The Ab Initio Based Theory Of Phase Transformations In Iron
The Transport Of Water In Subduction Zones
 Chapter 6 Phase Equilibria Ppt Video Online Download
Chapter 6 Phase Equilibria Ppt Video Online Download
 Phase Diagrams An Overview Sciencedirect Topics
Phase Diagrams An Overview Sciencedirect Topics
Stability And Properties Of Liquid Co Sub 2 Sub At High Pressure
Problem Set 10 Assigned November 8 2013 Due Friday November 15
The Iron Carbon Phase Diagram Ispatguru Com
Carbon Dioxide New World Encyclopedia
 Equilibrium P T Phase Diagram Of Boron Experimental Study And
Equilibrium P T Phase Diagram Of Boron Experimental Study And
 Spectroscopy Of Planetary Atmospheres In Our Galaxy Springerlink
Spectroscopy Of Planetary Atmospheres In Our Galaxy Springerlink
 Equation Of State For Solid Phase I Of Carbon Dioxide Valid For
Equation Of State For Solid Phase I Of Carbon Dioxide Valid For

0 Response to "Consider This Phase Diagram For Carbon Which Phases Are Present At The Upper Triple Point"
Post a Comment