Electron Dot Diagram For Methane
The ch 4 lewis structure is one of the most frequently tested lewis structures. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell.
 The Lewis Dot Structure For Ch4 Makethebrainhappy
The Lewis Dot Structure For Ch4 Makethebrainhappy
Electron dot structure valence electrons are represented by dots placed around the chemical symbol.

Electron dot diagram for methane. Learn what the lewis dot structure for ch4 is in this post by makethebrainhappy. Ch4 has 8 total valence electrons. Which of the.
Notice the lone pairs of electrons on the oxygen atoms are still shown. Learn what the lewis dot structure for ch4 is in this post by makethebrainhappy. Note that hydrogen atoms always go on the outside of a lewis dot structure.
Learn vocabulary terms and more with flashcards games and other study tools. Electrons are placed up to two on each side of the elemental symbol for a maximum of eight which is the number of electrons in a filled s and p shell. A step by step explanation of how to draw the ch4 lewis dot structure.
Drawing the lewis structure for ch 4 named methane requires only single bondsits one of the easier lewis structures to draw. Carbon has 4 and each h has 1 total 4. Lewis dot dragram for methane.
This is because they can share a maximum of two electrons. Log in sign up. Lewis dot symbols and lewis structures.
Carbon typically like to make 4 bonds because carbon has 4 valence e and it wants to get 8 total making four bonds will give it 8 total. Lewis structure of acetic acid. Drawing the lewis structure for ch 4.
However a more realistic view can be seen in the following models. For ch 4 you have a total of 8 total valence electrons. The following is a 3 d lewis structure for methane ch 4.
Acetic acid ch 3 cooh can be written out with dots indicating the shared electrons or preferably with dashes representing covalent bonds. First count total number of valence electrons. Methane ch4 1 methane ch4.
In fact the molar mass of methane is so minuscule that it is sometimes mentioned as a possible lifting gas because its density is less than that. Start studying chem exam. Log in sign up.
The overall lewis structure gives us a feel for how the atoms in a molecule are arranged in space. The lewis structure does not give a real 3 dimensional structure of a molecule but it is a really good first attempt. In the correct lewis structure for the methane ch4 molecule how many unshared electron pairs surround the carbon.
Methane for the ch4 lewis structure calculate the total number of valence electrons for the ch4 molecule ch4 has 8. Explains how to draw the lewis dot structure for ch 4 methane.
 Unit 2 Day 5 Representing Hydrocarbons Carbon Using Lewis
Unit 2 Day 5 Representing Hydrocarbons Carbon Using Lewis
Lewis Diagrams For Compound Formation
 How To Draw The Lewis Dot Structure Of A Molecule
How To Draw The Lewis Dot Structure Of A Molecule
 Electron Dot Formula Electron Dot Formula For H2o Cs2 O2 Hcl
Electron Dot Formula Electron Dot Formula For H2o Cs2 O2 Hcl
 Kossel Lewis Approach To Chemical Bonding Emedicalprep
Kossel Lewis Approach To Chemical Bonding Emedicalprep
:max_bytes(150000):strip_icc()/formaldehyde_LD-56a12a2c3df78cf772680353.png) Lewis Structure Example Problem Formaldehyde
Lewis Structure Example Problem Formaldehyde
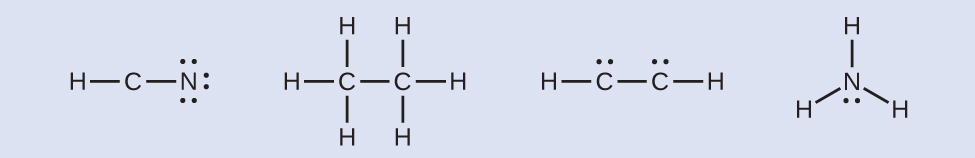 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
 Lewis Dot Structure And Polarity Of Ccl4 Carbon Tetrachloride
Lewis Dot Structure And Polarity Of Ccl4 Carbon Tetrachloride
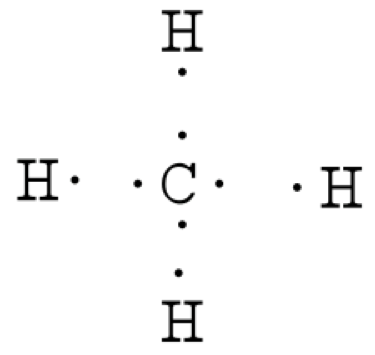 Images Of Methane Model Lewis Dot Structure Rock Cafe
Images Of Methane Model Lewis Dot Structure Rock Cafe
Lewis Dot Diagram For Hydrogen Sulfide Luxury H2s Sensor Diagram
 Lewis Structures Learn How To Draw Lewis Structures Albert Io
Lewis Structures Learn How To Draw Lewis Structures Albert Io
 Ch4 Dot Diagram Wiring Diagram Origin
Ch4 Dot Diagram Wiring Diagram Origin
 Lewis Dot Structure For Chlorine Atom Cl Youtube
Lewis Dot Structure For Chlorine Atom Cl Youtube
Lewis Diagrams For Compound Formation
Methane Diagram Prettier The Gallery For Methane Electron Dot
/Lewis-dot-58f78f405f9b581d5938e617.jpg)

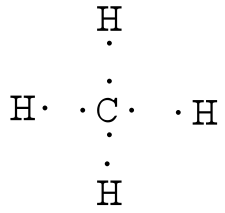



0 Response to "Electron Dot Diagram For Methane"
Post a Comment