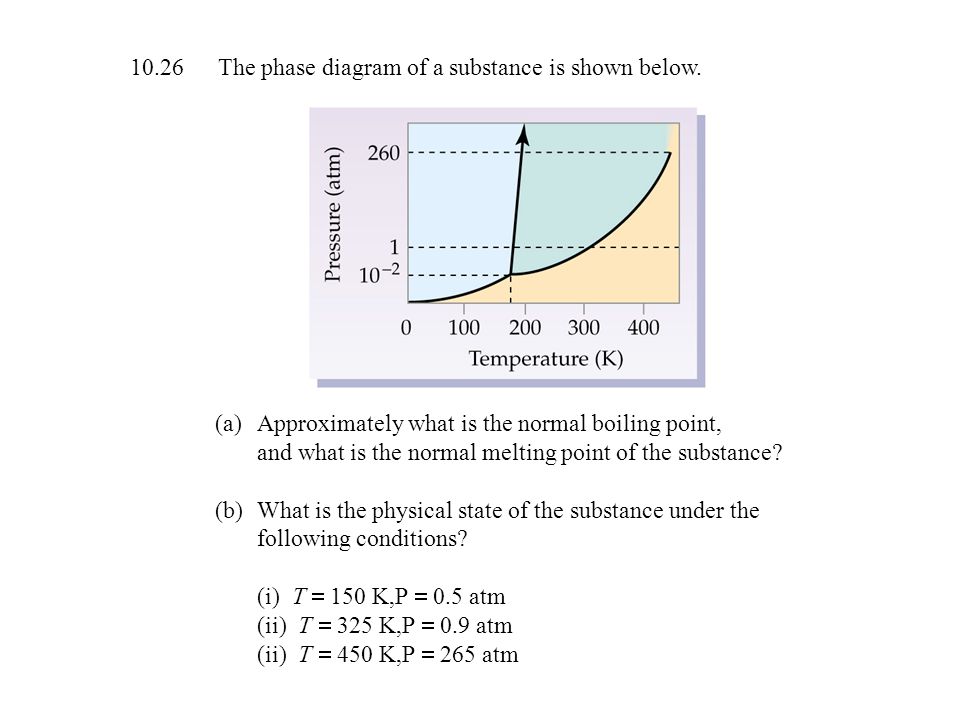
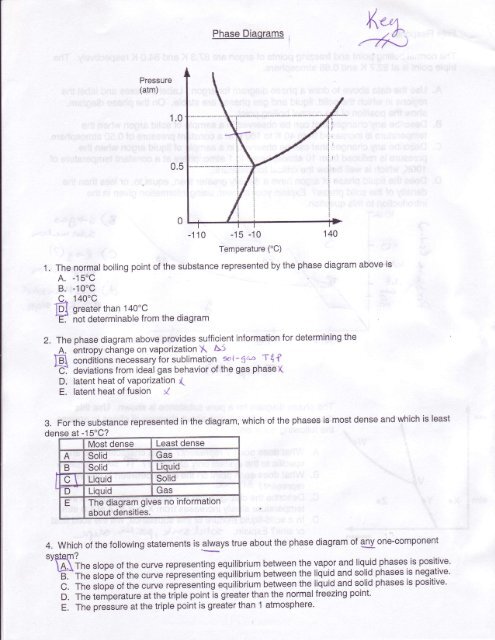
The Normal Boiling Point For The Substance In The Phase Diagram Below Is Approximately
Approximately what is the normal boiling point of the substance. Part a water vapor originally at 0005 atm and 05c is slowly compressed at constant temperature until the final pressure is 20 atm.
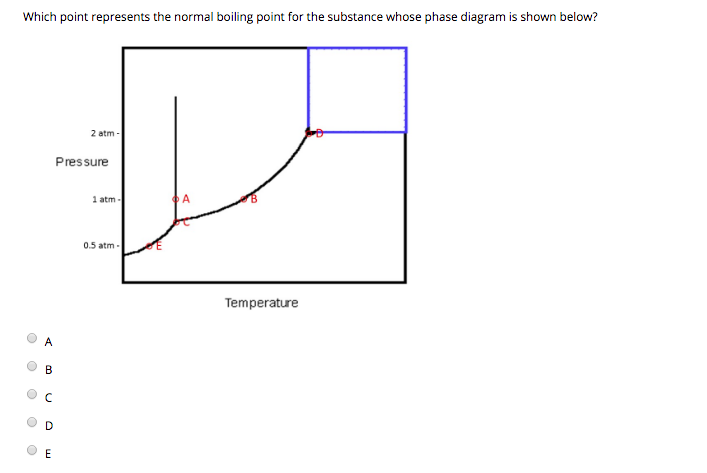 Solved Which Point Represents The Normal Boiling Point Fo
Solved Which Point Represents The Normal Boiling Point Fo
Deviations from ideal gas behavior of the gas phase d.
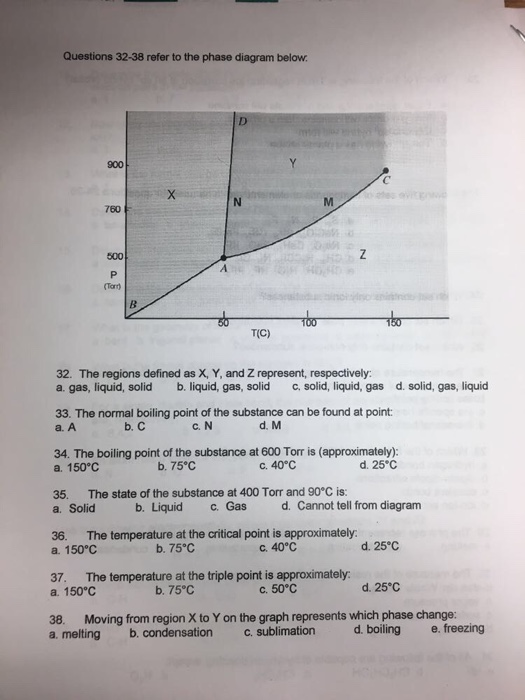
The normal boiling point for the substance in the phase diagram below is approximately. Imagine lowering the pressure on liquid water along the line in the diagram below. It represents the equilibrium between the liquid and gas phases. The line from a to b is the vapor pressure curve of the liquid.
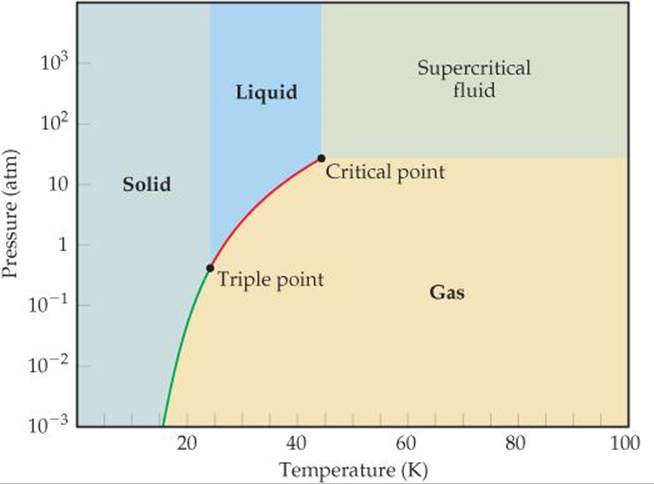
The phase diagram shows that the water would first freeze to form ice as it crossed into the solid area. When the pressure fell low enough the ice would then sublime to give water vapour. Gas liquid and solid.
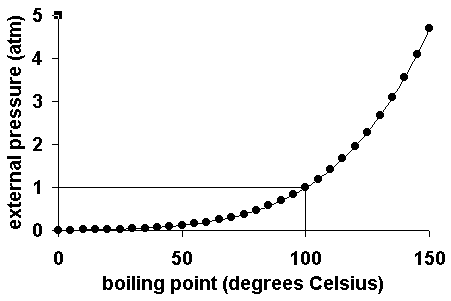
The normal boiling point of the substance represented by this phase diagram is approximately 2. Express your answer using two significant figures. Notice that the liquid vapor curve terminates at a temperature of 374 c and a pressure of 218 atm.
This liquid vapor curve separates the liquid and gaseous regions of the phase diagram and provides the boiling point for water at any pressure. Conditions necessary for sublimation c. Refer to the figure and describe all the phase changes that would occur in each of the following cases.
Express your answer using two significant figures. Express your answer using two significant figures. The figure below shows an example of a phase diagram which summarizes the effect of temperature and pressure on a substance in a closed containerevery point in this diagram represents a possible combination of temperature and pressure for the system.
Normal melting and boiling points. Figure 1124 general shape for a phase diagram of a system exhibiting three phases. B estimate the normal freezing point of the substance.
Phase diagrams of pure substances. The phase diagram of a hypothetical substance is shown below. The approximate normal boiling point of this substance is 300 k imagine a reaction that results in a change in both volume and temperature as shown in the diagram below.
Entropy change on vaporization b. The phase diagram of a substance is shown below. What is the triple point of this system.
Figure 1 a estimate the normal boiling point of the substance. This phase diagram provides sufficient information for determining the a. For example at 1 atm the boiling point is 100 c.
The point on this curve where the vapor pressure is 1 atm is the normal boiling point of the substance.
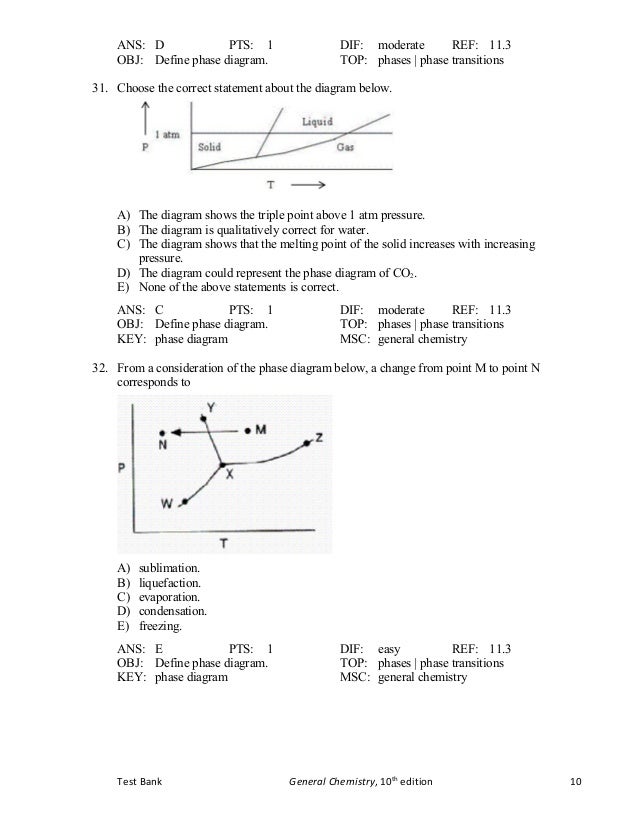 Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
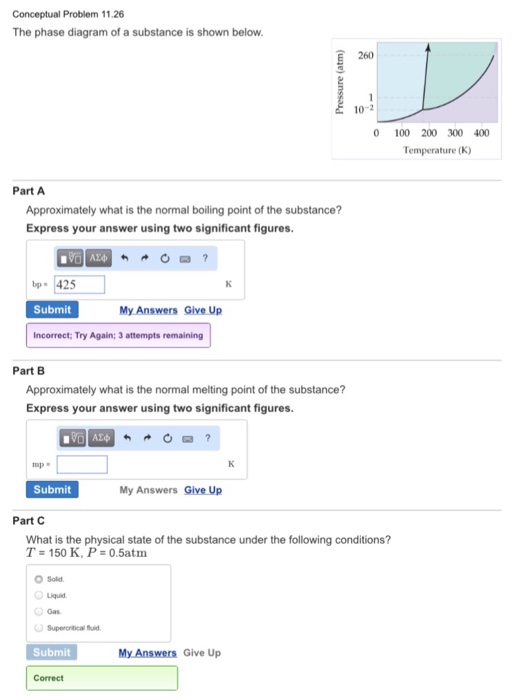
 Critical Point Thermodynamics Wikipedia
Critical Point Thermodynamics Wikipedia
Invariant Points And Gibbs Phase Rule
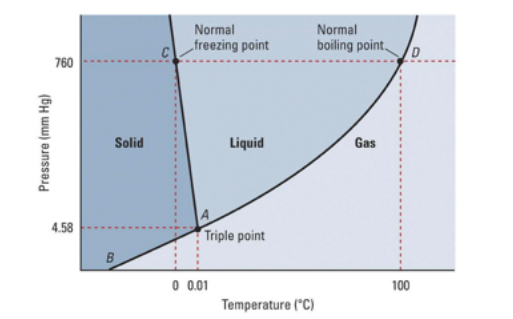
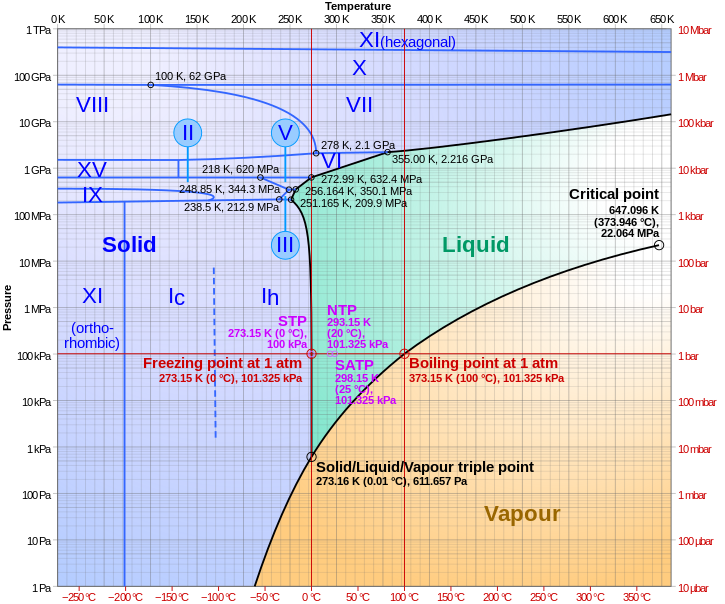
 Phase Diagram For Water Chemistry For Non Majors
Phase Diagram For Water Chemistry For Non Majors
 Exercises Liquids And Intermolecular Forces Chemistry The
Exercises Liquids And Intermolecular Forces Chemistry The
Quick Review Use The Phase Diagram Below To Answer The Pages 1
/phasediagram-56a129b35f9b58b7d0bca3ea.jpg) Critical Point Definition Chemistry
Critical Point Definition Chemistry
Physical Chemistry Examples Class For Second Year Students
 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
 He Phase Diagram 13 7 Stromoeko De
He Phase Diagram 13 7 Stromoeko De
 Ethyl Acetate Ch3co2ch2ch3is Commonly Used As A Solvent And Nail
Ethyl Acetate Ch3co2ch2ch3is Commonly Used As A Solvent And Nail

 Answer What Does Letter D Represent A Clutch Prep
Answer What Does Letter D Represent A Clutch Prep
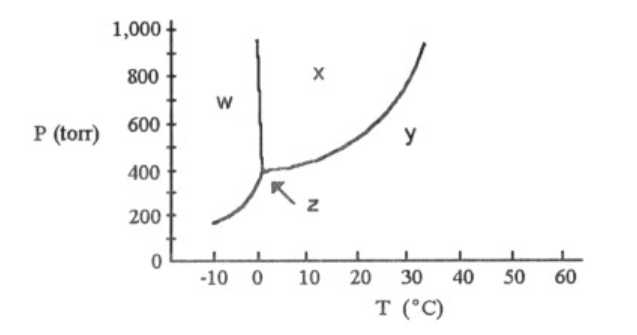 Solved According To The Phase Diagram Shown Above The No
Solved According To The Phase Diagram Shown Above The No
0 Response to "The Normal Boiling Point For The Substance In The Phase Diagram Below Is Approximately"
Post a Comment