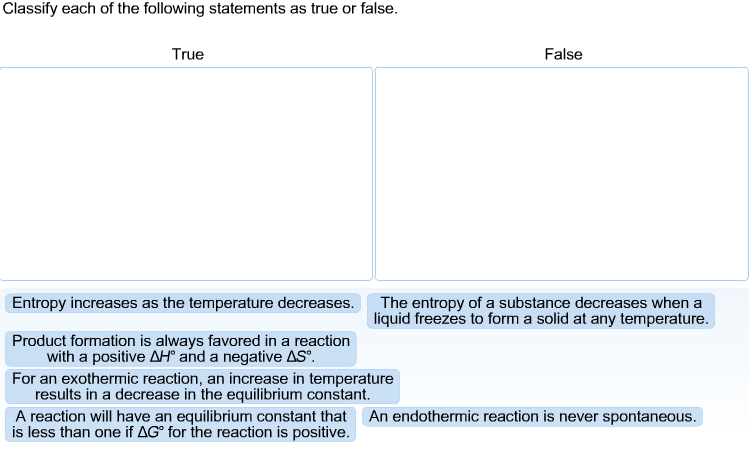
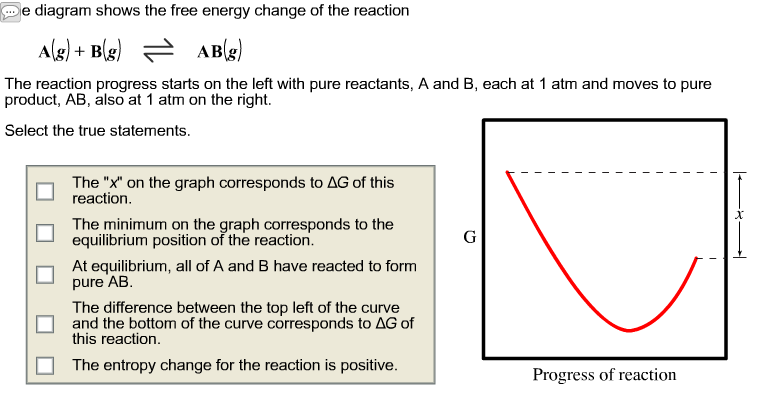
The Diagram Shows The Free Energy Change Of The Reaction
A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions.
 Shows The Change In Free Energy A And Log Likelihood B For The
Shows The Change In Free Energy A And Log Likelihood B For The
When talking about thermal dynamics and kinetics one of our best friends is going to be the free energy diagram.

The diagram shows the free energy change of the reaction. A what is the significance of the minimum in the plotb what does the quantity x shown on the right side of the diagram represent. The entropy change for the reaction is positive. 2 calculate the partial pressure of ag at equilibrium.
The reason free energy diagrams are important is because theyre going to serve as a summary of the thermal dynamics and kinetics of a reaction. The diagram shows the free energy change of the reaction ab the reaction progress starts on the left with pure reactants a and b each at 1 atm and moves to pure product ab also at 1 atm on the right. Reaction coordinate diagrams the mechanism of a reaction describes the various steps that are believed to occur as reactants are converted into products.
The reaction progress starts on the left with pure reactants a and b each at 1 atm and moves to pure product c also at 1 atm on the right. The diagram shows the free energy change of the reaction ag bg cg. Each at 1 atm and moves to pure product c also at 1 atm on the right.
Show transcribed image text the diagram shows the free energy change of the reaction ag bg rightarrow cg the reaction progress starts on the left with pure reactants a and b. Free energy and equilibrium. Place the statements in the appropriate place on the diagram.
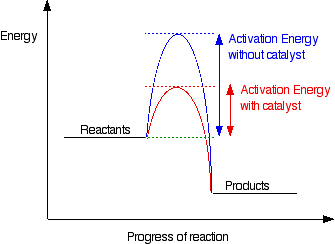
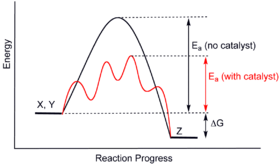
Show transcribed image text the diagram shows the free energy change of the reaction the reaction progress starts on the left with pure reactants a and b. It also shows the effect of a catalyst on the forward and reverse activation energy. However for endothermic reactions the reactants are drawn below the products.
The accompanying diagram shows how the free energy g changes during a hypothetical reaction ag bg cg on the left are pure reactants each at 1 atm and on the right is the pure product also at 1 atm. Energy diagrams depict the reaction progress versus energy. Assume that g subscript a 8850 jmol and g subscript b 12490 jmol 1calculate the value of the equilibrium constant for this reaction.
So its going to be essential that we learn how to interpret these correctly. Ag in equilibrium with bg at 25 degrees c. Catalysts lower activation energy so they decrease the size of the hump within the diagram itself.
At equilibrium all of a and b have reacted to form pure ab. In a reaction coordinate diagram the total energy of all species is plotted. Select the true statements.
For exothermic reactions the reactants are drawn above the products because their energy is greater. Spontaneous nonspontaneous q k q k. Each at 1 atm and moves to pure product ab also at 1 atm on the right.
 Solved Gdie Diagram Shows The Free Energy Change Of The R
Solved Gdie Diagram Shows The Free Energy Change Of The R
 Solved Gdie Diagram Shows The Free Energy Change Of The R
Solved Gdie Diagram Shows The Free Energy Change Of The R
 Shows The Change In Free Energy A And Log Likelihood B For The
Shows The Change In Free Energy A And Log Likelihood B For The
 Energy And Metabolism Biology For Non Majors I
Energy And Metabolism Biology For Non Majors I
Metamorphic Petrology Geology 102c
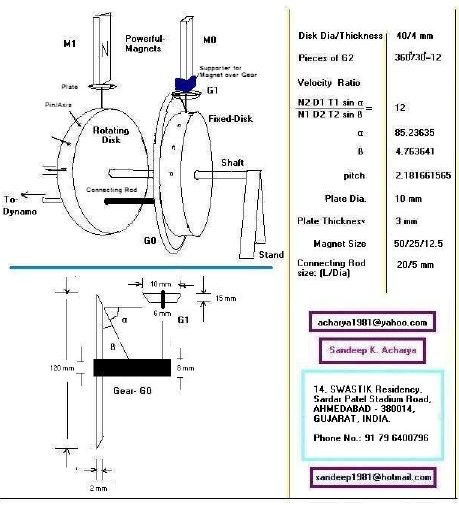 How To Build A Free Energy Magnetic Motor The Green Optimistic
How To Build A Free Energy Magnetic Motor The Green Optimistic
 Thermodynamics Reaction Coordinate Diagram Gibbs Free Energy
Thermodynamics Reaction Coordinate Diagram Gibbs Free Energy
How Much Energy Is Released In Atp Hydrolysis
Factors That Affect The Rate Of Reactions Introductory Chemistry
 Free Energy An Overview Sciencedirect Topics
Free Energy An Overview Sciencedirect Topics
Notes Chapter 17 Equilibrium Kinetics
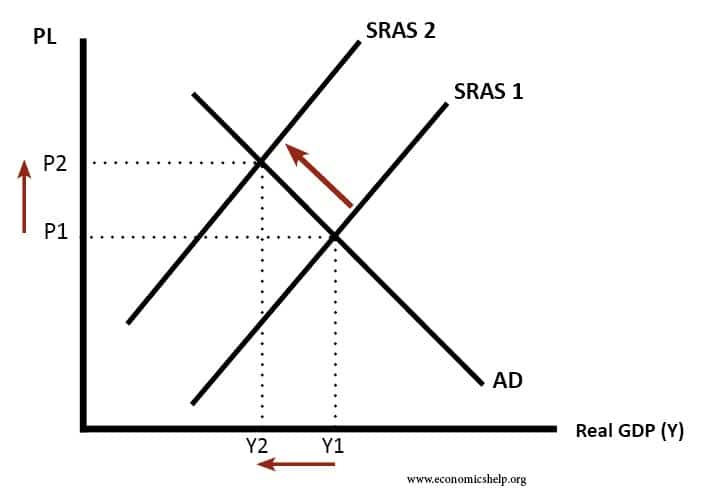 Cost Push Inflation Economics Help
Cost Push Inflation Economics Help
Gibbs Free Energy Changes Equation Calculations Reaction Feasibility
 A Look At Energy Profiles For Reactions Chemistry Libretexts
A Look At Energy Profiles For Reactions Chemistry Libretexts
Helmholtz And Gibbs Free Energies
Thermochemistry In Gaussian Gaussian Com

0 Response to "The Diagram Shows The Free Energy Change Of The Reaction"
Post a Comment