How To Calculate Bond Order From Molecular Orbital Diagram
Consider a simple example. Thus the bond order is two.
Molecular Orbitals In Carbon Monoxide
Using the mo diagram of no calculate the bond order.

How to calculate bond order from molecular orbital diagram. To obtain the bond order look at the molecular. Dihydrogen h 2 this mo diagram depicts the molecule h 2 with the contributing aos on. In molecular orbital theory we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital cancel the effect of one bond.
Hydrogen atoms have one electron in the s shell. Know that the higher the bond order the more stable the molecule. Dihelium he 2 the third diagram hypothesizes.
For instance the bond order of diatomic nitrogen nn is 3 and bond order between the carbon atoms in h hc h is also three. The bond order describes the stability of the bondthe molecular orbital provides an easy understanding of the concept of the bond order of a chemical bond. In molecular orbital theory.
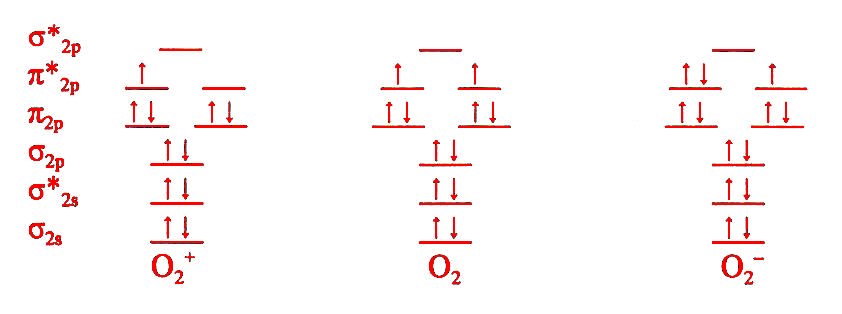
We can calculate the bond order in the o 2 molecule by noting that there are eight valence electrons in bonding molecular orbitals and four valence electrons in antibonding molecular orbitals in the electron configuration of this molecule. It quantifies the degree of covalent bonds between the atoms. Bond order is the number of chemical bonds between a pair of atoms.
Bond order indicates the stability of a bond. Compare it to no. In atoms electrons are located on atomic orbitals ao.
Bond order no. Determine bond order at a. Of electrons in anti bonding mo no.
Introduction to chemistry bond order in molecular orbital theory. In molecular orbital theory bond order is defined as half. Dihydrogen h 2 with an electron in the antibonding orbital.
If paramagnetism occurs due to unpaired electrons is no paramagnetic or diamagnetic. How to calculate bond order from molecular orbital diagram. In molecular orbital diagram we just need to calculate the number of electrons in anti bonding orbital and bonding orbital then we can use the formula in order to calculate bond order is.
Molecular orbital mo theory and the bond order. Of electrons in bonding mo 2. How to calculate bond order in chemistry know the formula.
In diatomic nitrogen nn for example the bond order is 3 while in acetylene hcch the bond order between the two carbon atoms is 3 and the ch bond order is 1. With one additional electron in an antibonding orbital 2b2 the bond order decreases by 12 relative to no.
 Molecular Orbital Energy Level Diagram Of Nitrogen Oxygen Youtube
Molecular Orbital Energy Level Diagram Of Nitrogen Oxygen Youtube
 Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
 Hybrid Orbitals And Molecular Orbital Theory Html Chm2045 F13 All
Hybrid Orbitals And Molecular Orbital Theory Html Chm2045 F13 All
 Molecular Orbital Mo Diagram Of N2 Youtube
Molecular Orbital Mo Diagram Of N2 Youtube
 Draw The Molecular Orbital Diagram Of Dioxygen And Calculate Bond
Draw The Molecular Orbital Diagram Of Dioxygen And Calculate Bond
S And P Molecular Orbitals A Spin Calculated For Complex 5 Using
 What Is The Bond Order Of Co Quora
What Is The Bond Order Of Co Quora
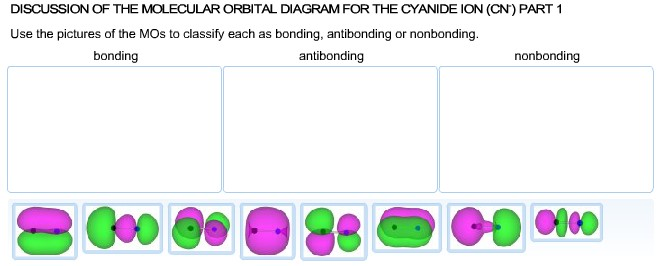 Solved Part 2 Calculate The Bond Order For Cyanide Ion P
Solved Part 2 Calculate The Bond Order For Cyanide Ion P
 Molecular Orbital Theory Bonding Antibonding Mo Bond Order
Molecular Orbital Theory Bonding Antibonding Mo Bond Order
M O Diagram For B2 Chemistry Community
 Write Molecular Orbital Configuration Of C2 Predict Magnetic
Write Molecular Orbital Configuration Of C2 Predict Magnetic
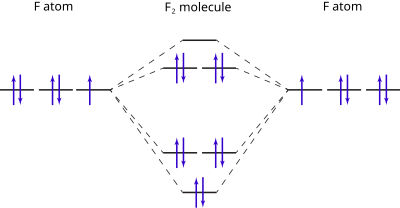 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
Molecular Orbital Theory Chemistry Encyclopedia Structure
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
Molecular Orbital Diagram For H2 Michaelhannan Co
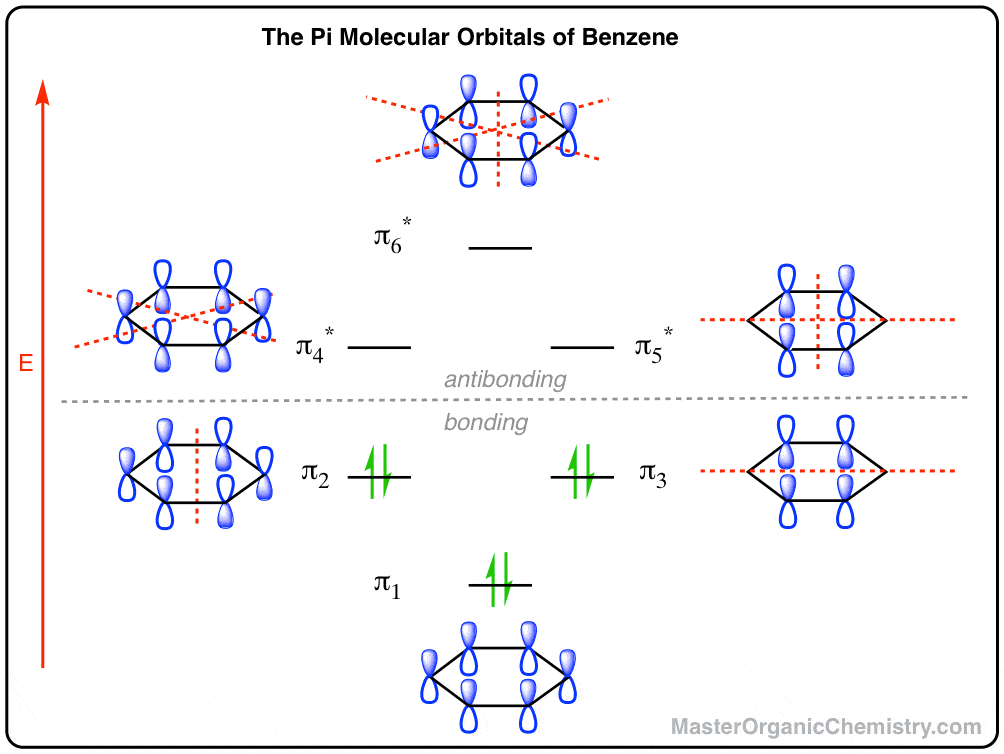 The Pi Molecular Orbitals Of Benzene Master Organic Chemistry
The Pi Molecular Orbitals Of Benzene Master Organic Chemistry
 Chemistry Molecular Structure 43 Of 45 Molecular Orbital Theory
Chemistry Molecular Structure 43 Of 45 Molecular Orbital Theory
 Energy Level Diagram For Molecular Orbitals Chemical Bonding And
Energy Level Diagram For Molecular Orbitals Chemical Bonding And
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict
Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict
How To Calculate Bond Order From Molecular Orbital Diagram Hf
Molecular Orbital Diagram Wikipedia
 Using The Mo Diagram Of No Calculate The Bond Order Compare It
Using The Mo Diagram Of No Calculate The Bond Order Compare It
0 Response to "How To Calculate Bond Order From Molecular Orbital Diagram"
Post a Comment