Which Diagram Shows A Pair Of Electrons That Have Opposite Spins
Follow report by jhubbell34 10152018. Each orbital can host two electrons of opposite spin.

To show the electron configuration for an atom what is the advantage of using an orbital notation compared to a dot structure.
Which diagram shows a pair of electrons that have opposite spins. Jump to navigation jump to search. Mc026 1jpg up arrow and down arrow consider the electron configuration. Which diagram shows the correct distribution of electrons in the electron shells of a helium atom.
Some questions will include multiple choice options to show you the options involved and other questions will just have the questions and corrects answers. Simply reveal the answer when you are ready to check your work. This collapses person 2s to downward imediantly.
Which diagram shows a pair of electrons that have opposite spins. You have reached the limit. Ask for details.
Mo diagrams depicting covalent left and polar covalent right bonding in a diatomic molecule. The electron configuration for helium he is shown below. In the experiment where there are two electrons and one is shot out to a person the experiment depends on the two having opposite spins one up one down though which has which is not determined until observation.
Orbital notation shows the spin of the electrons. The electron pair concept was introduced in a 1916 paper of gilbert n. Diagram of the outermost shell of fluorine atom.
Therefore two electrons or any other two fermions can have the same energy but only if they have opposite spins. Which diagram shows a pair of electrons that have opposite spins. Chemistry chapter 2 quiz 6.
For example if the position of a pair of electrons are described as 4px this means they are in the fourth energy level n4 and have a dumbbell shaped orbital orbital quantum number is p around the x axis third quantum number. So person 1 looks at theirs and sees its an upward spin. So the electrons occupying the same space must have opposite spin and so only two electrons can occupy the same orbital as a result of this as three electrons will results in two electrons having the same spin state.
According to paulis exclusion principle no more than one fermion can have the same spin in the same space. But the spin is also part of the state and it has two values up and down. These two electrons in this orbital as stated above would have opposite spins.
In chemistry an electron pair or a lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. This is what happens in the shell model of the atoms.
Neutrons Repel Each Other Through The Nuclear Strong Force As Do
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
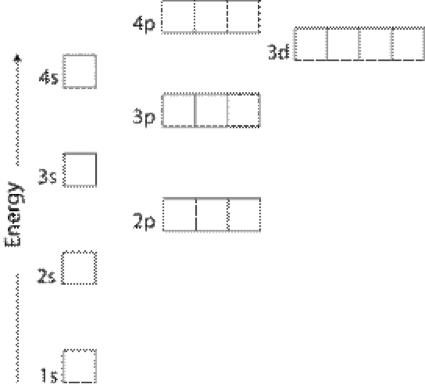 Electron Configurations Orbitals Energy Levels And Ionisation
Electron Configurations Orbitals Energy Levels And Ionisation
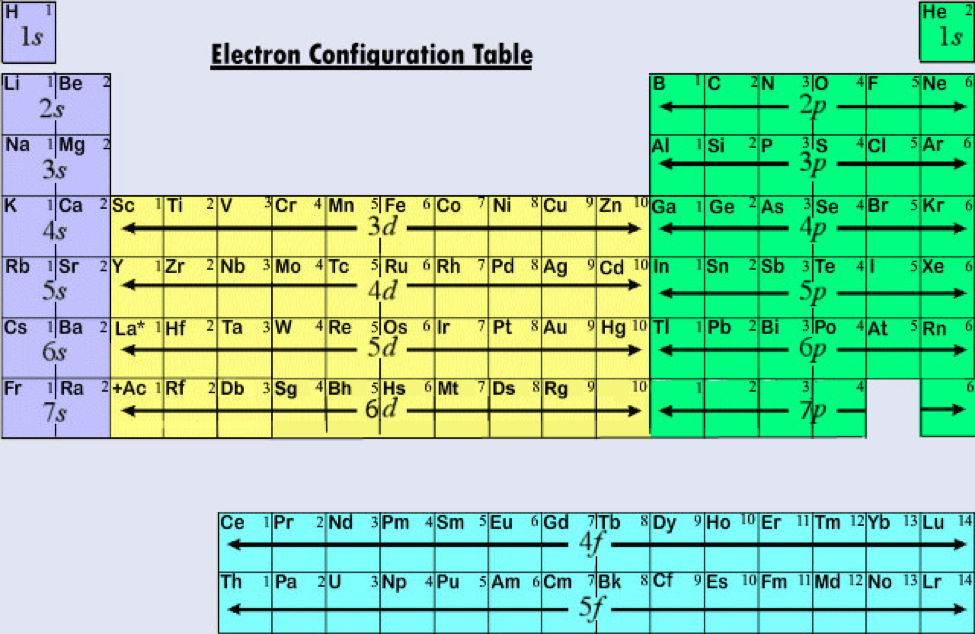 Electron Configurations Orbitals Energy Levels And Ionisation
Electron Configurations Orbitals Energy Levels And Ionisation
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
Orbital Mapping Of Energy Bands And The Truncated Spin Polarization

 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 A Two Pairs Of Resonant Landau Levels With Opposite Spins Of
A Two Pairs Of Resonant Landau Levels With Opposite Spins Of
 Dublin Schools Lesson Orbital Diagrams And Electron Configurations
Dublin Schools Lesson Orbital Diagrams And Electron Configurations
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Hybrid Atomic Orbitals Chemistry For Majors
Hybrid Atomic Orbitals Chemistry For Majors
 A Child S Puzzle Has Helped Unlock The Secrets Of Magnetism
A Child S Puzzle Has Helped Unlock The Secrets Of Magnetism
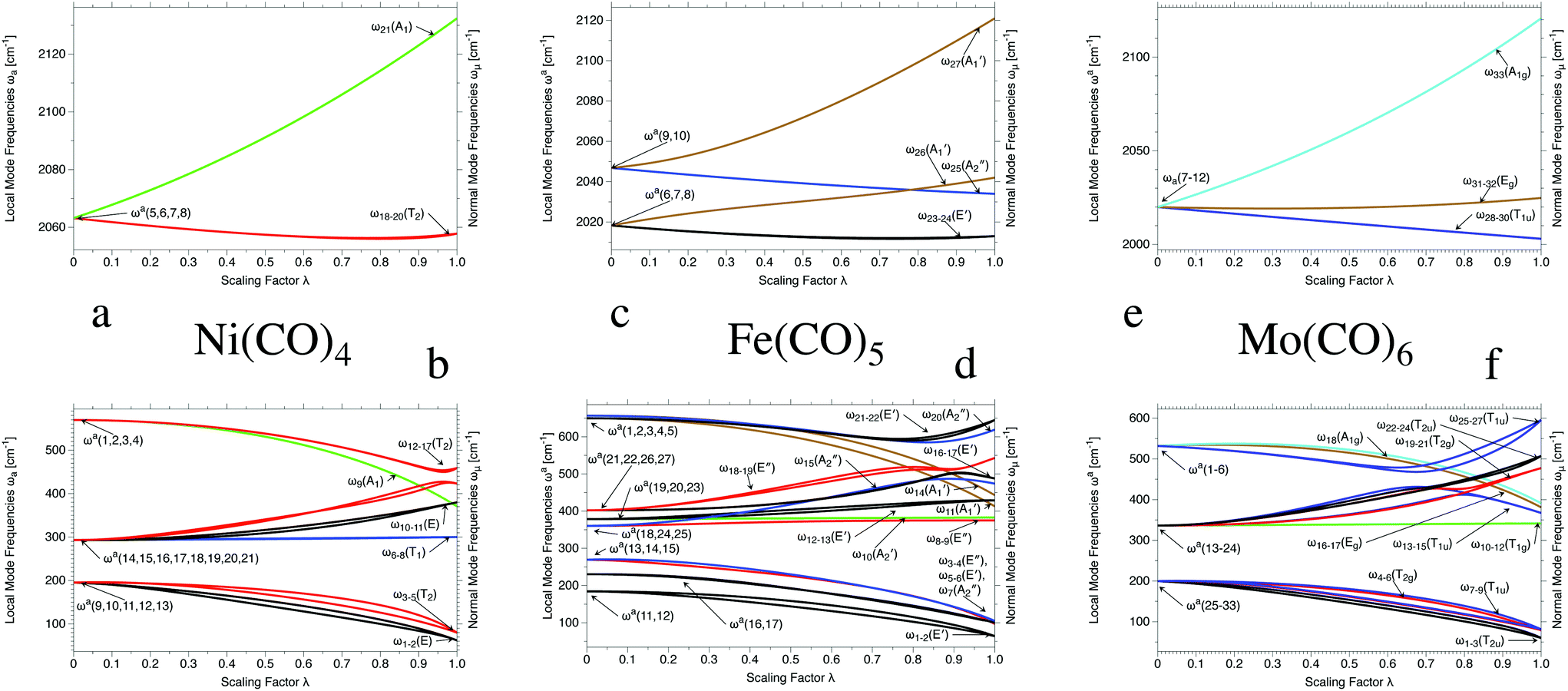 Generalization Of The Tolman Electronic Parameter The Metal Ligand
Generalization Of The Tolman Electronic Parameter The Metal Ligand
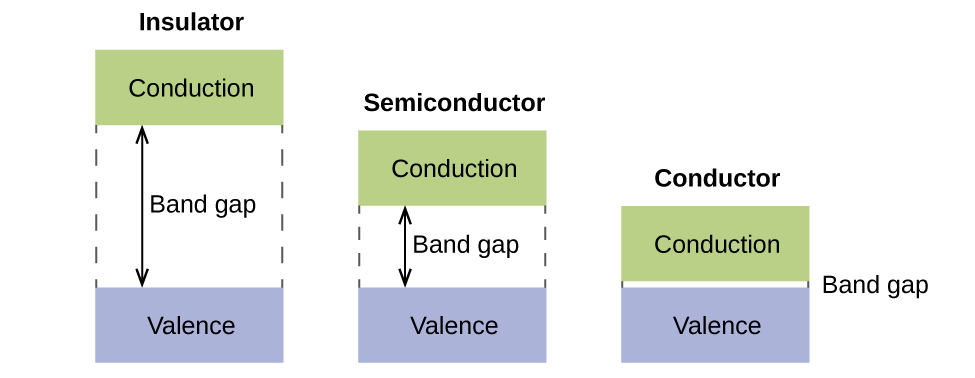
![]() Chapter2 Energy Bands And Charge Carriers In Semiconductors
Chapter2 Energy Bands And Charge Carriers In Semiconductors
 Quantum Numbers Atomic Orbitals And Electron Configurations
Quantum Numbers Atomic Orbitals And Electron Configurations

0 Response to "Which Diagram Shows A Pair Of Electrons That Have Opposite Spins"
Post a Comment