How To Calculate Bond Order From Mo Diagram
The bond order in sulfur dioxide for example is 15 the average of an s o single bond in one lewis structure and an so double bond in the other. The bond order shows the number of chemical bonds present between a pair of atoms.
 Nptel Chemistry And Biochemistry Introductory Inorganic Chemistry
Nptel Chemistry And Biochemistry Introductory Inorganic Chemistry
Bond order is the number of chemical bonds between a pair of atoms.

How to calculate bond order from mo diagram. Of electrons in bonding mo 2. 2b1 is the pi2px antibonding mo. In molecular orbital diagram we just need to calculate the number of electrons in anti bonding orbital and bonding orbital then we can use the formula in order to calculate bond order is.
In molecular orbital theory we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital cancel the effect of one bond. Bond order indicates the stability of a bond. Quick overview of what the labels correspond to what mos.
For instance the bond order of diatomic nitrogen nn is 3 and bond order between the carbon atoms in h hc h is also three. 1b1 is the pi2px bonding mo. Of electrons in anti bonding mo no.
The bond order describes the stability of the bondthe molecular orbital provides an easy understanding of the concept. If we draw the lewis structure for no3 we may find out that its a resonance structure with one double bond and two single bonds. 3a1 is the sigma2pz bonding mo but its relatively nonbonding with respect to oxygen.
A single covalent bond has a bond order of one. 1a1 is the sigma2s bonding mo. So the bond order is 4.
A triple covalent bond three and so on. For example o2 has a double bond and has a bond order of 2. In its most basic form the bond order is the number of bonded electron pairs that hold two atoms together.
2b2 is the pi2py antibonding mo. 1b2 is the pi2py bonding mo. In diatomic nitrogen nn for example the bond order is 3 while in acetylene hcch the bond order between the two carbon atoms is 3 and the ch bond order is 1.
2a1 is the sigma2s antibonding mo. A double covalent bond a bond order of two. Consider how atoms come together into molecules.
4a1 is the sigma2pz antibonding mo. Basically you can calculate the bond order by counting the bonds with the help of the lewis structure. Bond order no.
Molecular Orbital Diagram Wikipedia
 11 1 Chapter 11 Theories Of Covalent Bonding Theories Of Covalent
11 1 Chapter 11 Theories Of Covalent Bonding Theories Of Covalent
Calculating Bond Order Chemistry Community
 Introduction To Inorganic Chemistry Molecular Orbital Theory
Introduction To Inorganic Chemistry Molecular Orbital Theory
 By Writing Molecular Orbital Configuration For No Co O2 Molecules
By Writing Molecular Orbital Configuration For No Co O2 Molecules
 Introduction To Inorganic Chemistry Molecular Orbital Theory
Introduction To Inorganic Chemistry Molecular Orbital Theory
 Nptel Chemistry And Biochemistry Introductory Inorganic Chemistry
Nptel Chemistry And Biochemistry Introductory Inorganic Chemistry
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 How To Build Molecular Orbitals Chemistry Libretexts
How To Build Molecular Orbitals Chemistry Libretexts
 Orbitals How To Rationalise With Mo Theory That Co Is A Two
Orbitals How To Rationalise With Mo Theory That Co Is A Two
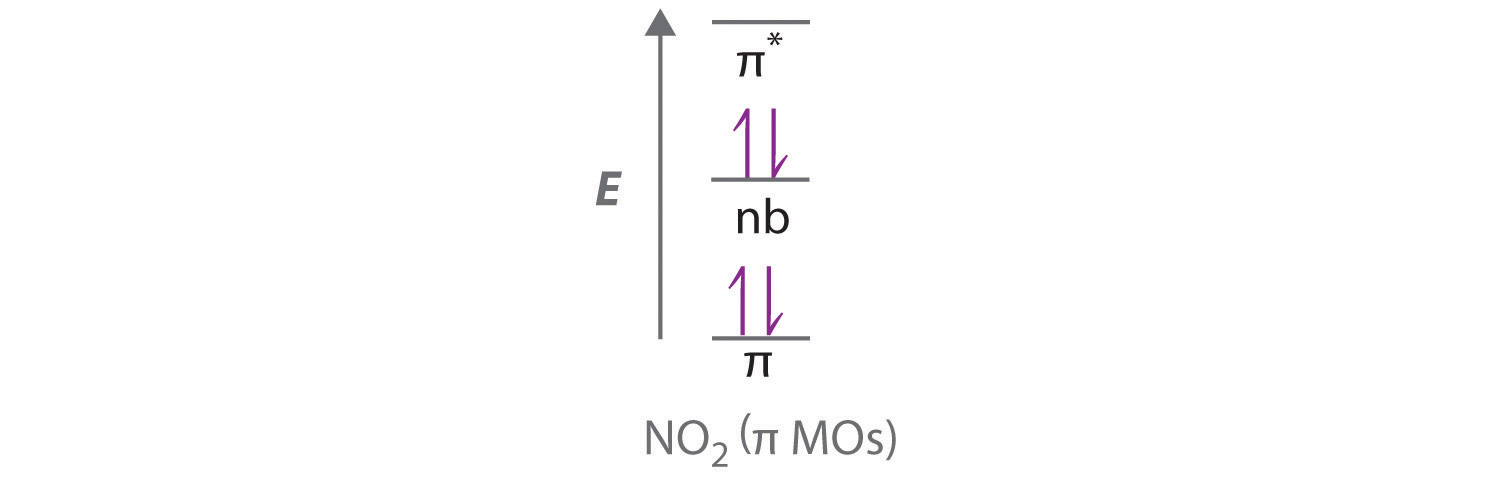 4 11 Multiple Bonds In Mo Theory Chemistry Libretexts
4 11 Multiple Bonds In Mo Theory Chemistry Libretexts
 How To Build Molecular Orbitals Chemistry Libretexts
How To Build Molecular Orbitals Chemistry Libretexts
 By Writing Molecular Orbital Configuration For No Co O2 Molecules
By Writing Molecular Orbital Configuration For No Co O2 Molecules
M O Diagram For B2 Chemistry Community
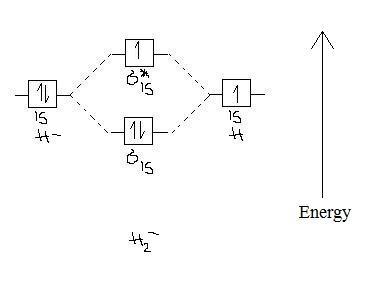 2 3b Mo Theory Of Bonding In H Chemistry Libretexts
2 3b Mo Theory Of Bonding In H Chemistry Libretexts
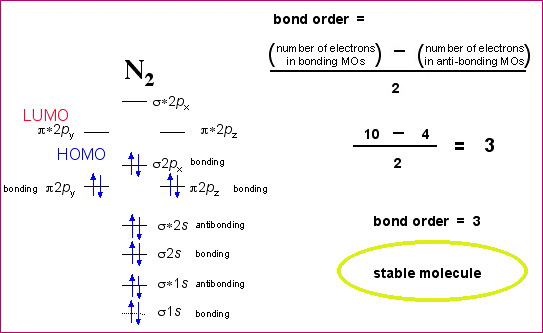
 Calculate The Bond Order Of N2 And N2 And Predict Its Magnetic
Calculate The Bond Order Of N2 And N2 And Predict Its Magnetic
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
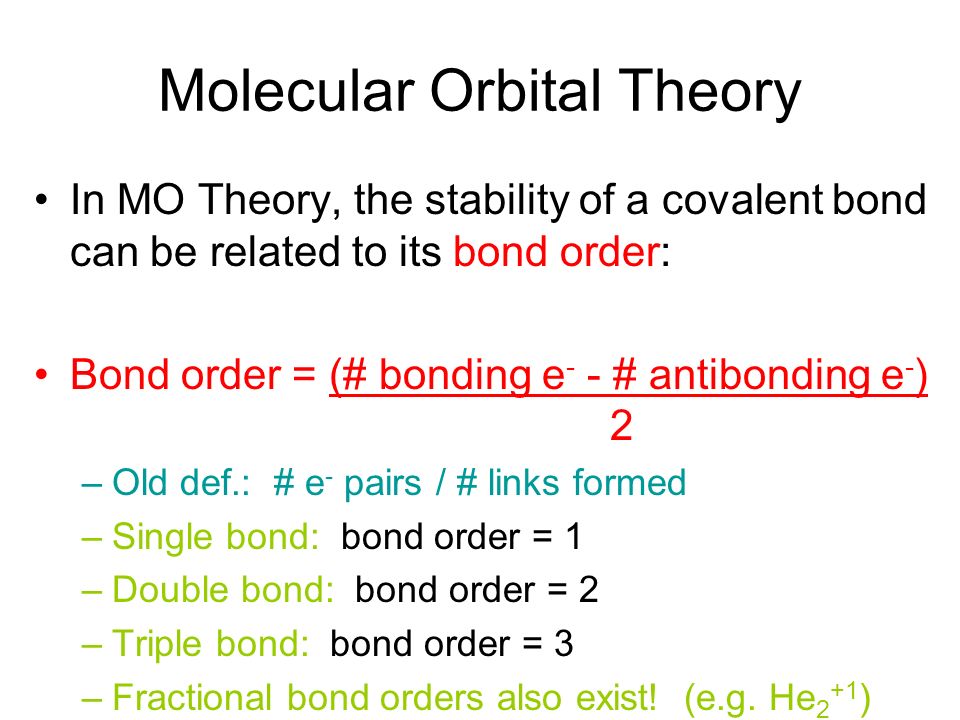 Chapter 10 Covalent Bond Theories Ppt Video Online Download
Chapter 10 Covalent Bond Theories Ppt Video Online Download
 Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia

0 Response to "How To Calculate Bond Order From Mo Diagram"
Post a Comment