Energy Diagram For Exothermic Reaction
Energy reactants products exothermic reactions the reactants have more potential energy than the products have. Energy diagrams for endothermic and exothermic reactions.
What Is Difference Between Endothermic And Exothermic Reaction If
An energy level diagram shows whether a reaction is exothermic or endothermic.
Energy diagram for exothermic reaction. Energy profile diagrams for endothermic and exothermic reactions. The reaction shown by the second diagram is more exothermic. The extra energy is released to the surroundings.
You can start with a generic potential energy diagram for an exothermic reaction. Heat of reaction is given the symbol ah and is usually measured in kilojoules kj. There is a greater difference in energy between the reactants and products.
In other words the products are less stable than the reactants. The energy values points on the hyper surface along the reaction coordinate result in a 1 d energy surface a line and when plotted against the reaction coordinate energy vs reaction coordinate gives what is called a reaction coordinate diagram or energy profile. δh total energy content of products total energy content of reactants h products h.
It also shows the effect of a catalyst on the forward and reverse activation energy. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small.
The green arrow is longer. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants the is the activation energy than it is released when the products are formed. More on pe diagrams.
Energy must be input in order to raise the particles up to the higher energy level. Reactants products energy. So the activation energy is the minimum amount of energy required for a reaction to take place.
In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. The amount of heat energy released or absorbed during a chemical reaction is called the heat of reaction.
Endothermic reactions take in energy and the temperature of the surroundings decreases.
Pitatspay Endothermic Activation Energy Diagram Of Enzymes

 C Draw An Energy Diagram Of The Above Reaction Assuming An
C Draw An Energy Diagram Of The Above Reaction Assuming An
Kinetics The Activation Energy Endothermic Reaction Energy Diagram
Energy Diagram Endothermic And Exothermic Reaction Luxury Enthalpy
Comparing Endothermic And Exothermic Potential Energy Diagrams
 Thermochemistry An Endothermic Reaction Carolina Com
Thermochemistry An Endothermic Reaction Carolina Com
 L O To Recognise What An Endothermic And Exothermic Reaction Is And
L O To Recognise What An Endothermic And Exothermic Reaction Is And
Energy Diagram Labeled Beautiful How Can I Represent An Exothermic
 Analyzing Energy With A Reaction Coordinate Diagram Study Com
Analyzing Energy With A Reaction Coordinate Diagram Study Com
 Definition Of Activation Energy Chegg Com
Definition Of Activation Energy Chegg Com
Reaction Energy Profiles Activation Energy Exothermic Endothermic
Energy Diagram For Exothermic Reaction Kejomoro Fresh Ideas
 Potential Energy Diagram Endothermic And Exothermic Energy Etfs
Potential Energy Diagram Endothermic And Exothermic Energy Etfs
Gcse Chemistry What Are Energy Level Diagrams What Is The
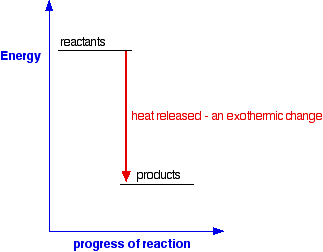 Chemical Energetics An Introduction
Chemical Energetics An Introduction
Endothermic Reaction Energy Diagram Luxury Chemistry Mysteries
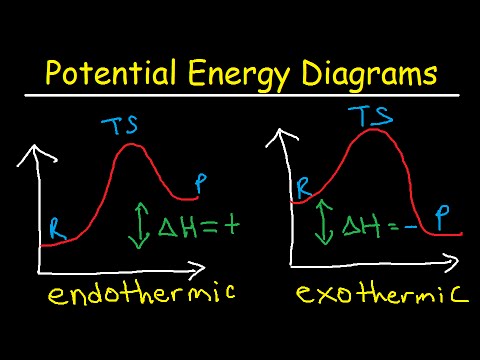 Potential Energy Diagrams Chemistry Catalyst Endothermic
Potential Energy Diagrams Chemistry Catalyst Endothermic

0 Response to "Energy Diagram For Exothermic Reaction"
Post a Comment