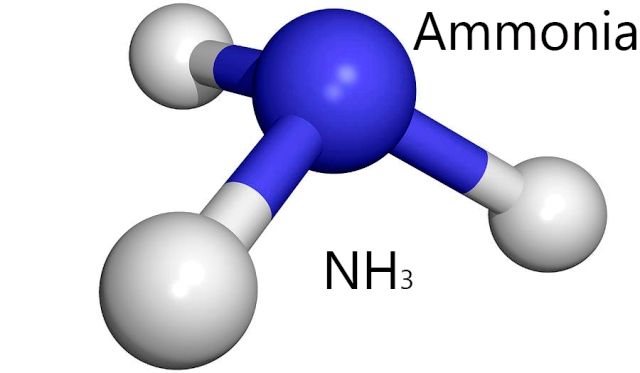
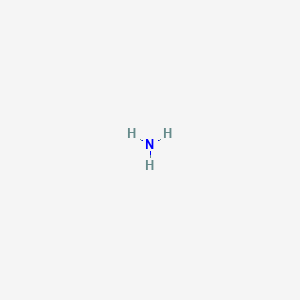
Which Of The Following Represents The Lewis Dot Diagram Of Ammonia Nh3
One molecule of hydrogen cyanide hcn is made up of one hydrogen atom one carbon atom and one nitrogen atom shown below. Nh3 commonly known as ammonia is arranged as a t shaped molecule with nitrogen at its center and three hydrogen atoms at its extremities.
 The Lewis Dot Structure For H2o Makethebrainhappy
The Lewis Dot Structure For H2o Makethebrainhappy
I also go over hybridization and bond angle.

Which of the following represents the lewis dot diagram of ammonia nh3. On the periodic table nitrogen is in group 5 or 15 so it has 5 valence electrons and then hydrogen is in group 1. That is that explains the basic character of ammonia. Alternatively a dot method can be used to draw the nh 3 lewis structure.
Lewis dot diagram for nh3. The lewis structure for nh3 is one of the most common lewis structures to show up on. In the space provided below draw electron dot diagrams for the following molecules.
Five plus 3 a total of 8 valence electrons. Which of the following represents the lewis dot diagram of ammonia nh3. Lewis structure of nh 3.
Each hydrogen atom is covalently bonded to the nitrogen via an electron pair and another pair of electrons is attached to the nitrogen atoms outer shell. Are you sure you want to delete this answer. We also have a handy video on the 5 things you need to know for general chemistry.
Were going to do the lewis structure for nh3. 1 sodium na 1 valence electron put 1 dot 2 chlorine cl 7 valence electrons put a dot on each side and then start pairing up if na and cl pair up they would form an ionic bond transfer of electrons and nonmetal bonded to metal. Diagram b hydrogen cyanide is poisonous liquid that has a faint almond like smell.
Lewis dot diagram nh3. We show two ways to draw the nh3 lewis structure ammonia. It has one valence electron but we have 3 hydrogens so lets mutiply that by 3.
Hydrogen h2 ammonia nh3 and methane ch4. 3 years ago. Ammonia or nitrogen trihydride.
Mimi 1 decade ago. Remember that the dots represent the valence electrons. Make sure that each atom in the molecules have 8 valence electrons except hydrogen which has only 2.
A step by step explanation of how to write the lewis dot structure for nh3 ammonia or nitrogen trihydride. I quickly take you through how to draw the lewis structure of ammonia nh3. The lewis structure is used to represent bonding in a molecule whether that be covalent or ionic.
 Cs2 Lewis Structure How To Draw The Lewis Structure For Cs2 Youtube
Cs2 Lewis Structure How To Draw The Lewis Structure For Cs2 Youtube
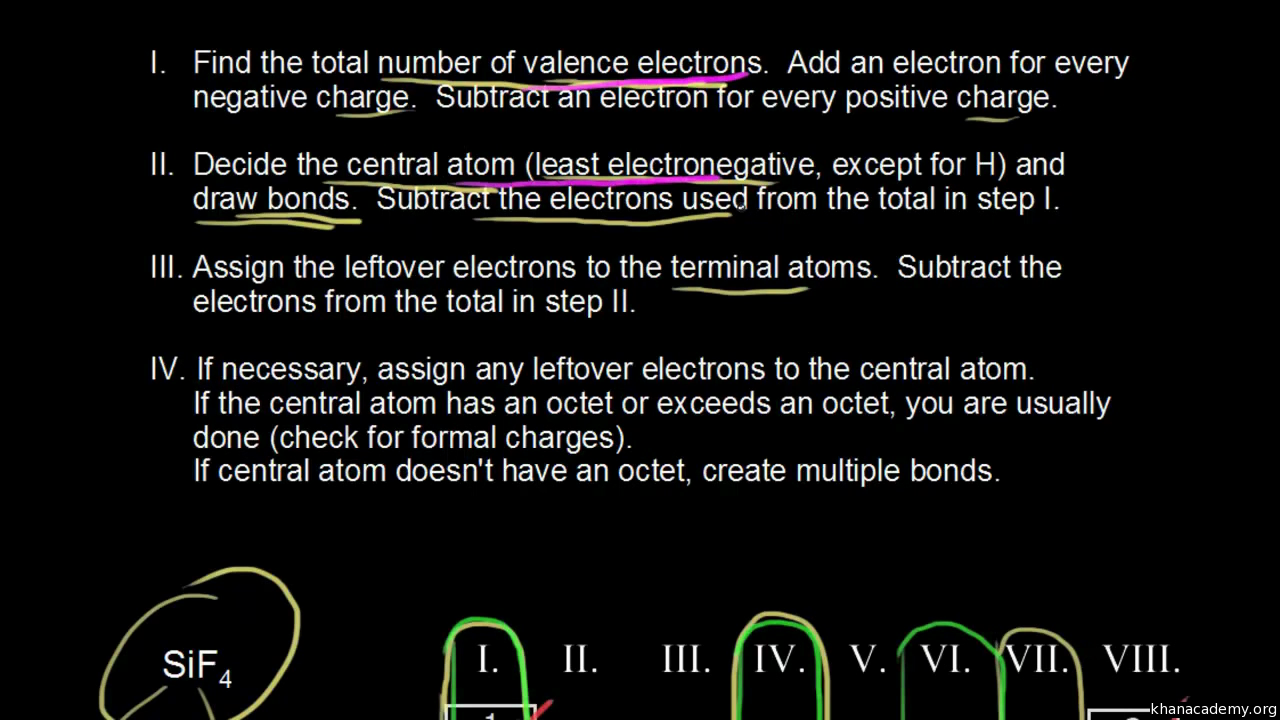 Drawing Dot Structures Video Khan Academy
Drawing Dot Structures Video Khan Academy
 Nh4cl Lewis Dot Structure Ammonium Chloride Youtube
Nh4cl Lewis Dot Structure Ammonium Chloride Youtube
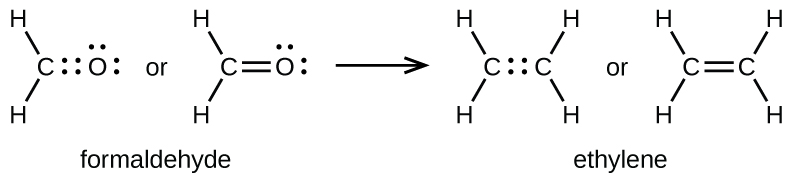 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
 The Lewis Dot Structure For Nh4 Makethebrainhappy
The Lewis Dot Structure For Nh4 Makethebrainhappy
 Nh4 Lewis Structure How To Draw The Dot Structure For Nh4
Nh4 Lewis Structure How To Draw The Dot Structure For Nh4
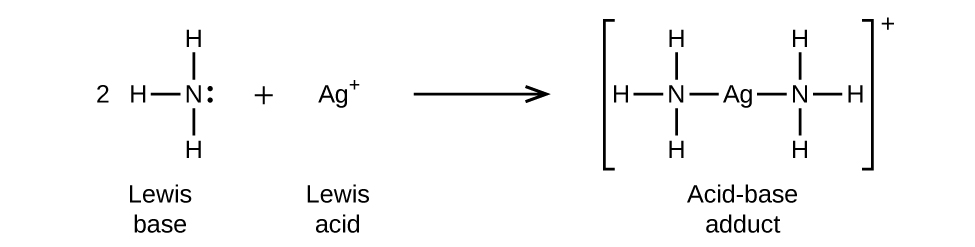 15 2 Lewis Acids And Bases Chemistry
15 2 Lewis Acids And Bases Chemistry
Co Ordinate Dative Covalent Bonding
Gcse Chemistry Covalent Bonding In An Ammonia Molecule What Is
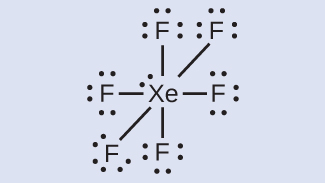 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
 The Lewis Dot Structure For Nh3 Makethebrainhappy
The Lewis Dot Structure For Nh3 Makethebrainhappy
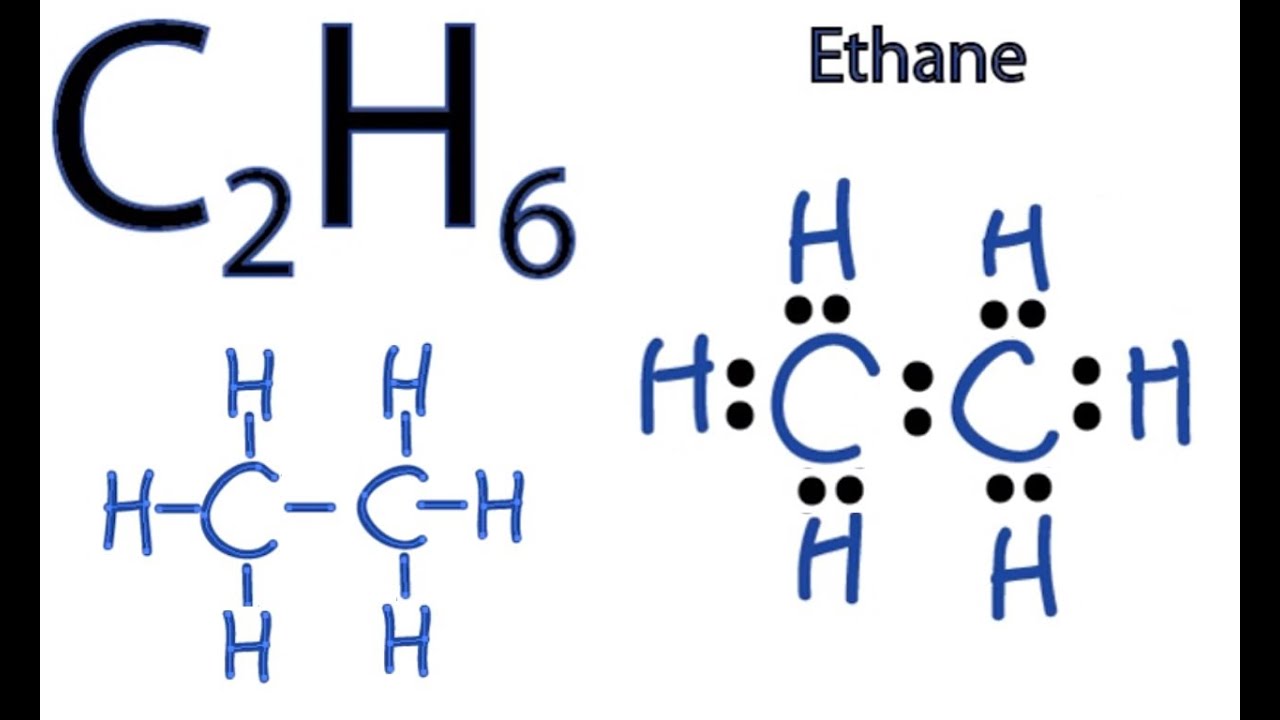 C2h6 Lewis Structure How To Draw The Dot Structure For C2h6 Youtube
C2h6 Lewis Structure How To Draw The Dot Structure For C2h6 Youtube
 Lewis Structure Of N2 Nitrogen Gas Youtube
Lewis Structure Of N2 Nitrogen Gas Youtube
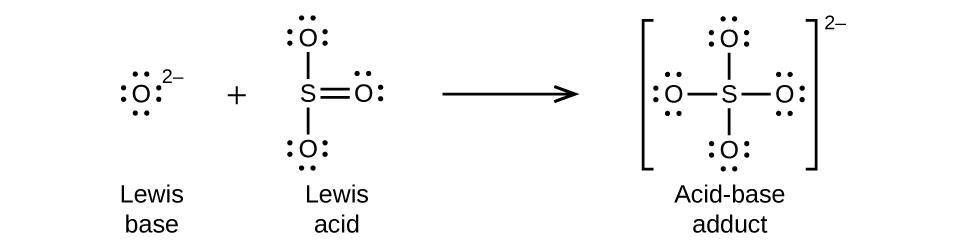 15 2 Lewis Acids And Bases Chemistry
15 2 Lewis Acids And Bases Chemistry
Gcse Chemistry Covalent Bonding In An Ammonia Molecule What Is
 So2 Lewis Structure How To Draw The Lewis Structure For So2
So2 Lewis Structure How To Draw The Lewis Structure For So2
 What Is The Lewis Structure Of N2 Socratic
What Is The Lewis Structure Of N2 Socratic
 How To Draw The Nh4 2so4 Lewis Dot Structure Ammonium Sulfate
How To Draw The Nh4 2so4 Lewis Dot Structure Ammonium Sulfate



0 Response to "Which Of The Following Represents The Lewis Dot Diagram Of Ammonia Nh3"
Post a Comment