Which Diagram Is A Bomb Calorimeter
Temperature of the water. A bomb calorimeter is a device that measures the heat given off or taken in by a reaction.
A bomb calorimeter measures heat for liquid products only.
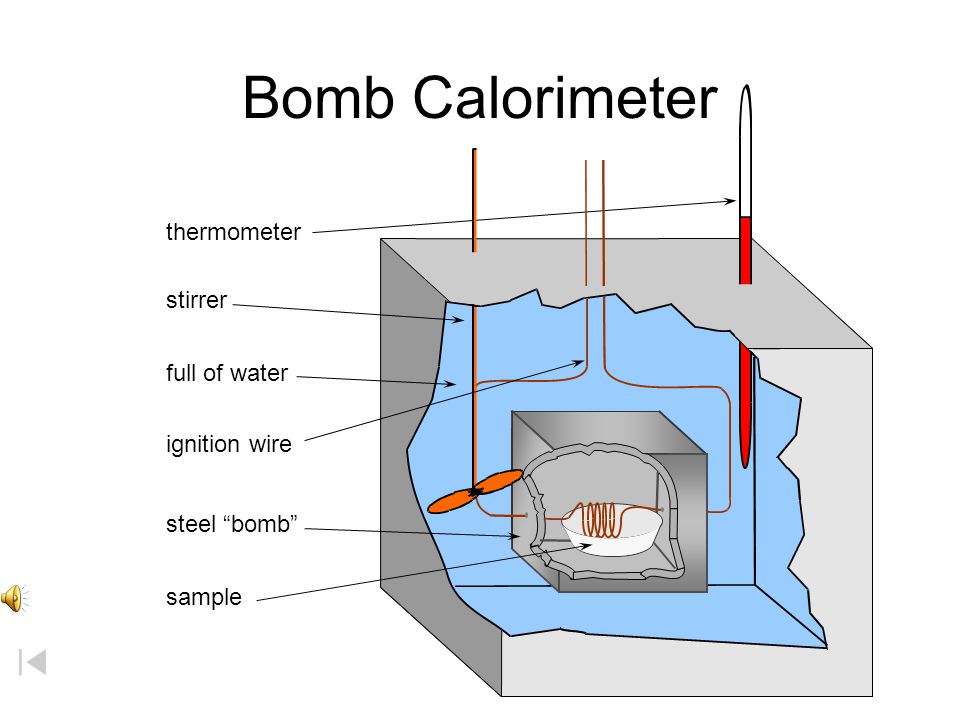
Which diagram is a bomb calorimeter. The calorimeter has a mass of 2000 kg and a specific heat of 295 jg c. A bomb calorimeter is used for measuring energy released in a combustion reaction. How does a bomb calorimeter work.
Electrical energy is used to ignite the fuel. In the human body the reaction takes place over a series of many intermediate reactions gradually releasing energy along the way. A bomb calorimeter can measure heat gain or loss in gaseous reactions but is not useful for reactions that occur at high pressures and temperatures.
In a bomb calorimeter the reaction takes place quickly. Bomb calorimeter a device for measuring the energy content of material. The parr bomb is a bomb calorimeter a type of constant volume calorimeter as opposed to typical styrofoam cup calorimeters which are constant pressure calorimeters at least in theory.
When the air is escaping through the copper tube it will also heat up the water outside the tube. This reaction takes place in a water bath so that the water absorbs the energy released and we can measure how much its temperature rises accordingly. A parrtm model 1108 oxygen combustion vessel as used in this experi ment.
The temperature of the calorimeter rises from 200c to 300c. 30th sep 2016 147 pm 20th feb 2017 in short the process of a calorimeter involves measuring the heat of a fuel sample when burned under stable temperature conditions to evaluate the heating energy of the fuel sample. The quantity of heat that is required to raise the samples temperature by 1c or kelvin a 120 g sample of propane c3h8 is burned in a bomb calorimeter.
The temperature of the water bath can be monitored via thermometer. A 120 g sample of propane c3h8 is burned in a bomb calorimeter. The temperature of the calorimeter rises from 200c to 300c.
The sample is burned in an oxygen rich atmosphere inside a sealed chamber that is surrounded by a jacket containing a known volume of water. Overall however the reactions are the same and the amount of energy released is comparable. The rise in temperature of the water is recorded and used to calculate the amount of heat produced.
The calorimeter has a mass of 2000 kg and a specific heat of 295 jg c. A bomb calorimeter has a separate chamber to hold substances and can even measure heat gain or loss for reactions that do not occur in water. As the fuel is burning it will heat up the surrounding air which expands and escapes through a tube that leads the air out of the calorimeter.
Alright so lets talk about a bomb calorimeter. Inside the calorimeter is a vessel in which the reaction occurs surrounded by a water bath.
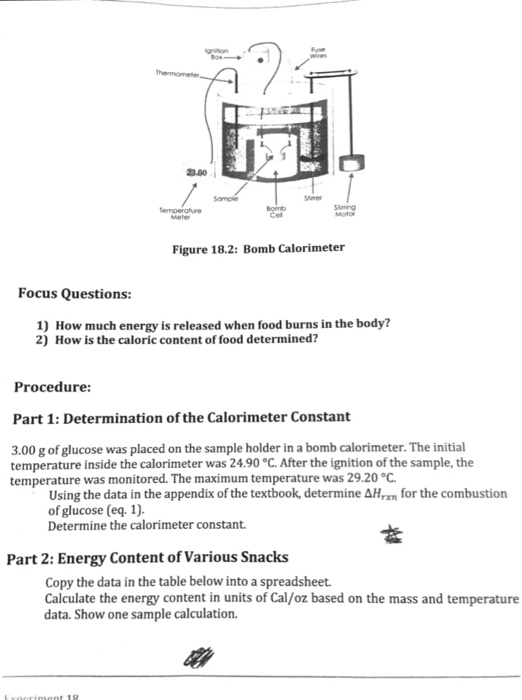
 Schematics Of The Ogamaseiko Adiabatic Bomb Calorimeter Download
Schematics Of The Ogamaseiko Adiabatic Bomb Calorimeter Download
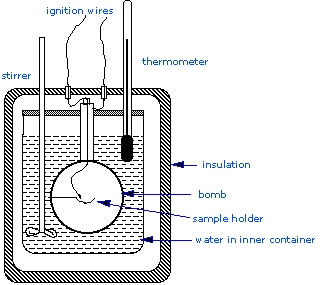 Constant Volume Calorimetry Chemistry Libretexts
Constant Volume Calorimetry Chemistry Libretexts
 Determination Of Calorific Value Using Bomb Calorimeter Youtube
Determination Of Calorific Value Using Bomb Calorimeter Youtube
:max_bytes(150000):strip_icc()/cross-section-of-a-typical-bomb-calorimeter--141482586-5aa68ad4642dca00367be3f3.jpg) Coffee Cup And Bomb Calorimetry
Coffee Cup And Bomb Calorimetry
Bomb Calorimeter Of Thermodynamics In Chemistry Class 11
Mercury S Help Desk Physical Chemistry Lab Notes
A Level Experimental Methods For Determining Enthalpy Changes
Nitrogen Triiodide The Study And Stabilisation Of An Explosive
Experiment 1 Adiabatic Bomb Calorimeter
 Aqa Biology A Level Bomb Calorimetry By 03margolism Teaching
Aqa Biology A Level Bomb Calorimetry By 03margolism Teaching
Adfs Bluebottle Files Chemistry Mainproj
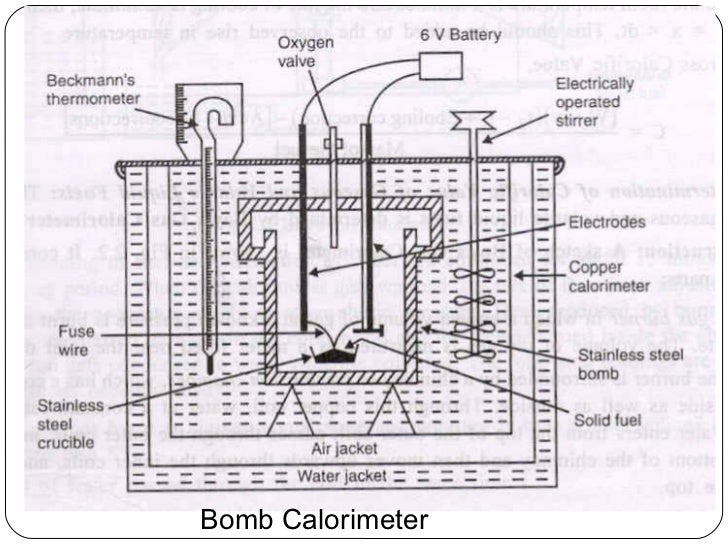


0 Response to "Which Diagram Is A Bomb Calorimeter"
Post a Comment