In This Phase Diagram For Water Indicate The Direction That The Solid Liquid And Liquid Gas
Assume 100 dissociation for electrolytes. The simplest phase diagrams are pressuretemperature diagrams of a single simple substance such as water.
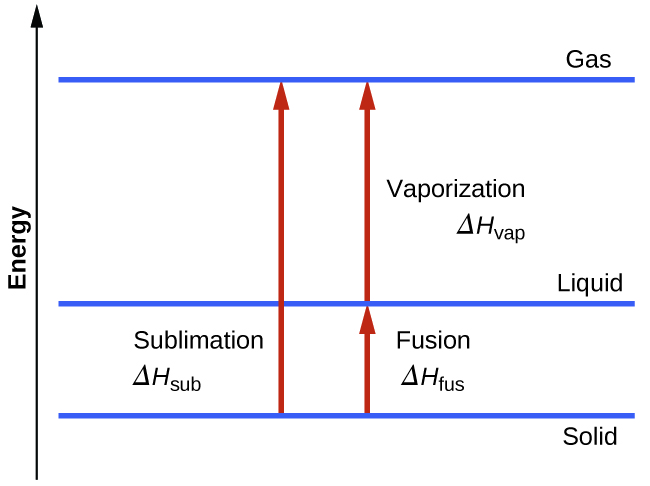 10 3 Phase Transitions Chemistry
10 3 Phase Transitions Chemistry
In this phase diagram for water indicate the direction that the solid liquid and liquid gas coexistence lines will move after the addition of solute.
In this phase diagram for water indicate the direction that the solid liquid and liquid gas. It includes a brief discussion of solubility curves. This page looks at the phase diagram for mixtures of salt and water how the diagram is built up and how to interpret it. The axes correspond to the pressure and temperature.
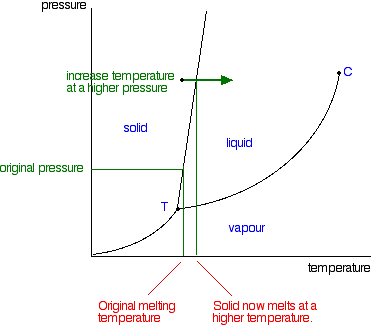
The triple point is the point on the phase diagram where the lines of equilibrium intersect the point at which all three distinct phases of matter solid liquid gas coexist. Phase diagrams phases of matter and phase transitions. When moving between solid to gas phases the material undergoes sublimation.
A semipermeable membrane separates two aqueous solutions at 20 c. In this phase diagram for water indicate the direction that the solid liquid and liquid gas coex. An increase in temperature will move the substance to the right on the phase diagram into the gas portion of the diagram.
In the opposite direction gas to solid phases the material undergoes deposition. Phase diagram in this phase diagram which is typical of most substances the solid lines represent the phase boundaries. And at 3 it would be a vapour a gas.
The triple point is the pace on a phase diagram where all three lines converge. For each of the following cases name the solution into which a net flow of water if any will occur. Under the set of conditions at 1 in the diagram the substance would be a solid because it falls into that area of the phase diagram.
If your institution is not listed please visit our digital product support community. At that point the substance exists as a mixture of gas liquid and solid all in equilibrium with one another. When moving in the opposite direction liquid phase to solid phase the material is freezing.
The phase diagram shows in pressuretemperature space the lines of equilibrium or phase boundaries between the three phases of solid liquid and gas. In this phase diagram for water indicate the direction that the solid liquid and liquid gas coexistence lines will move after the addition of solute. At 2 it would be a liquid.
 Vapour Pressure An Overview Sciencedirect Topics
Vapour Pressure An Overview Sciencedirect Topics
Reification Of The Present Kairos Laetus In Praesens Org
 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
 Pdf Solids Liquids And Gases Under High Pressure
Pdf Solids Liquids And Gases Under High Pressure

 Liquid Chemistry Properties Facts Britannica Com
Liquid Chemistry Properties Facts Britannica Com
Iron Carbon Phase Diagram A Review See Callister Chapter 9
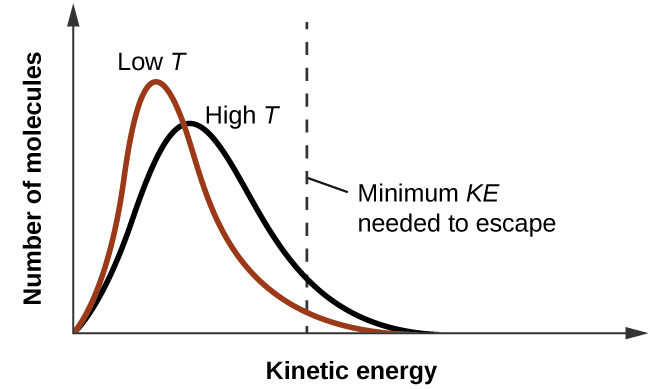 10 3 Phase Transitions Chemistry
10 3 Phase Transitions Chemistry
Magnetic And Electric Effects On Water
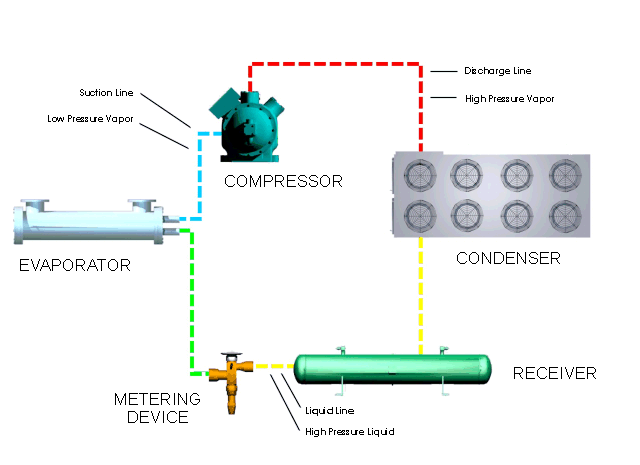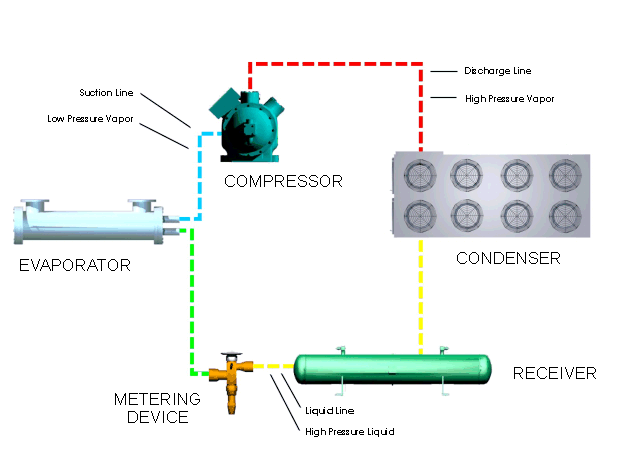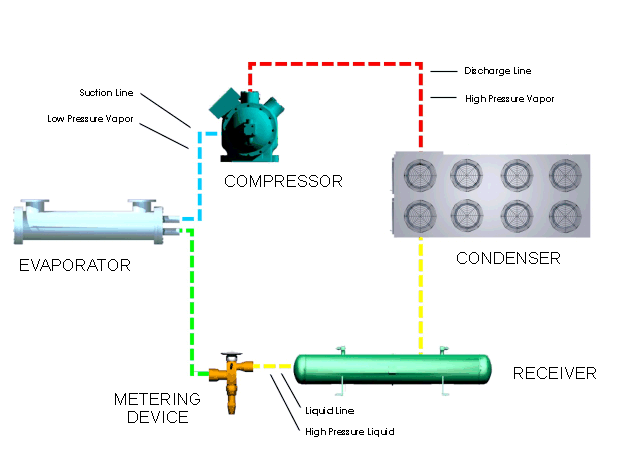 Refrigeration Principles And How A Refrigeration System Works Berg
Refrigeration Principles And How A Refrigeration System Works Berg

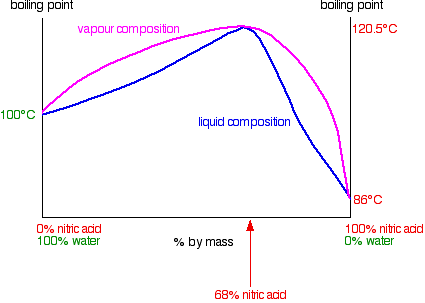
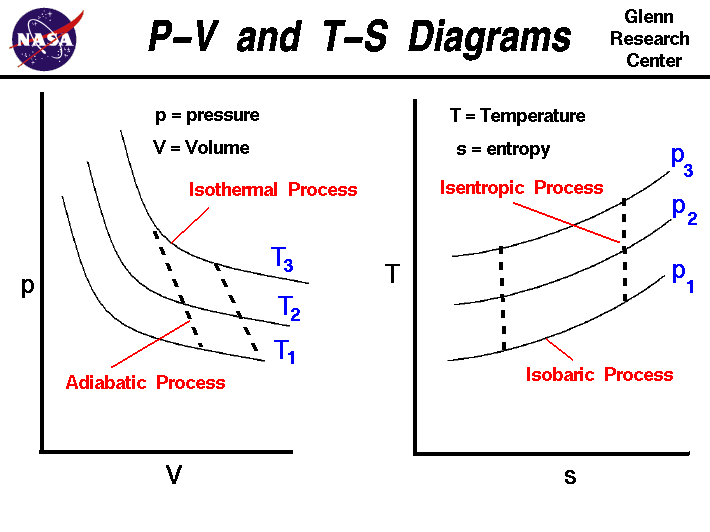
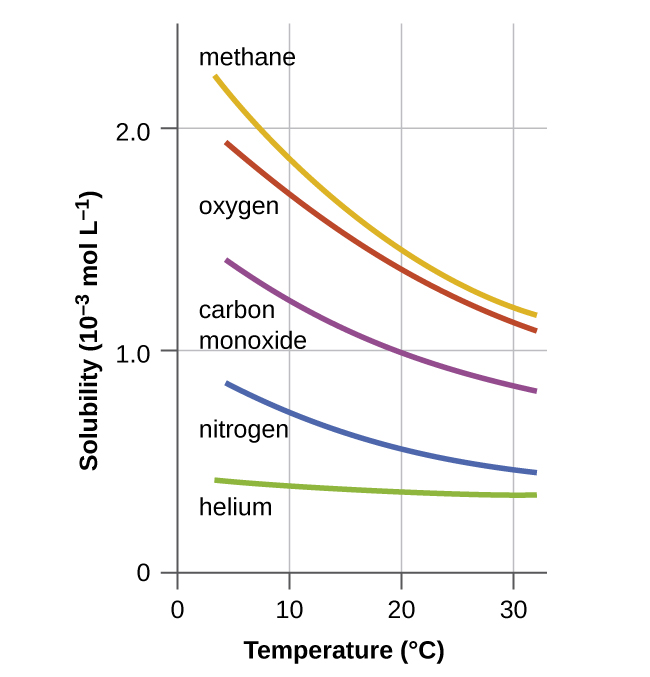
0 Response to "In This Phase Diagram For Water Indicate The Direction That The Solid Liquid And Liquid Gas"
Post a Comment