Orbital Diagram For F
Three rules are useful in forming orbital diagrams. These names together with the value of n are used to describe the electron configurations of atoms.
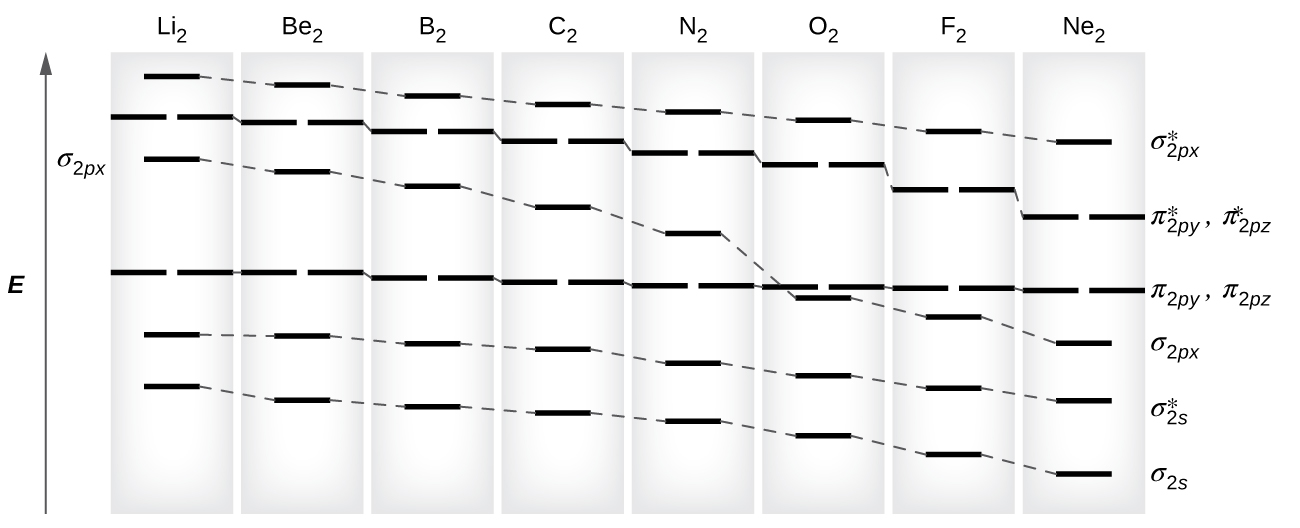 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
321 describe how schrodinger advanced the understanding of atomic structure.
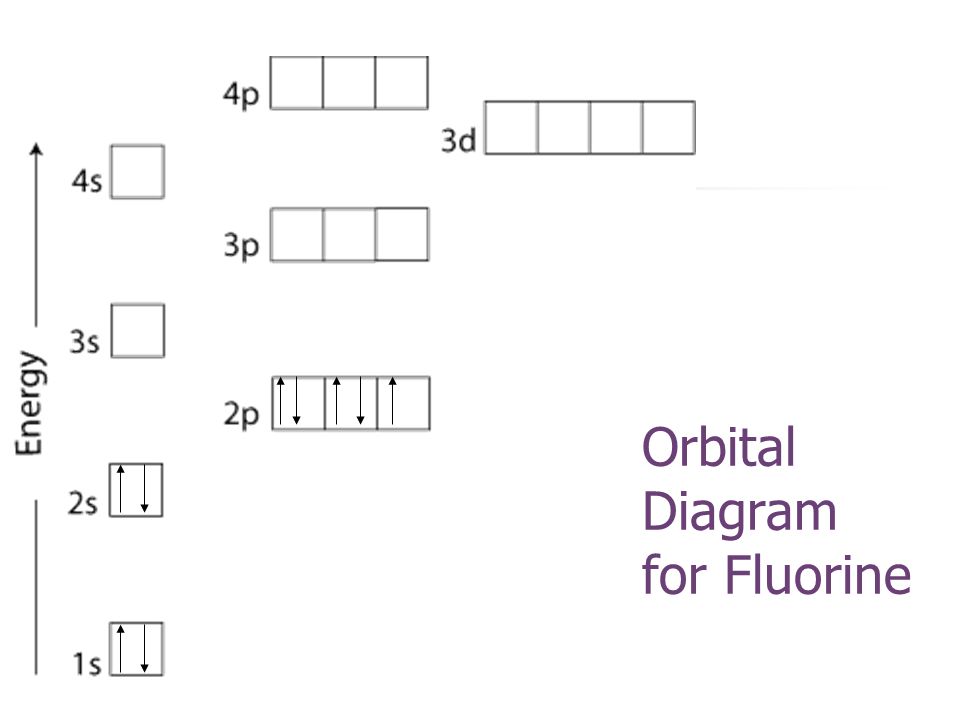
Orbital diagram for f. The remaining five electrons will go in the 2p orbital. According to the auf bau principle each electron occupies the lowest energy orbital. The simple names s orbital p orbital d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ 0 1 2 and 3 respectively.
Therefore the f electron configuration will be 1s 2 2s 2 2p 5. In an orbital filling diagram the individual orbitals are shown as circles or squares and orbitals within a sublevel are drawn next to each other horizontally. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital.
An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. Orbital diagrams and electron configurations. The pauli exclusion principle says that only two electrons can fit into an single orbital.
Many times it is necessary to see all the quantum numbers in an electron configuration this the purpose of the orbital diagram. 1s22s22p63s23p64s23d4 the orbital diagram above is formatted in such a manner as to place the various orbital types at different energy levels. Define orbital electron cloud.
Since 1s can only hold two electrons the next 2 electrons for f go in the 2s orbital. Orbital diagrams are pictorial descriptions of the electrons in an atom. Explain how the quantum mechanical model is based upon the idea that electrons travel in waves.
An orbital diagram naturally leads to the writing of an electron configuration. The aufbau principle the pauli exclusion principle and hunds rule. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular.
The electron configuration for chromium is. In addition to listing the principle quantum number n and the subshell ell the orbital diagram shows all the different orientations and the spin of every electron. Orbital diagrams are a visual way to show where the electrons are located within an atom.
Orbital diagrams must follow 3 rules.
Chemistry The Central Science Chapter 6 Section 9
 Parameters Used For Alloy Design The D F Orbital Energy Levels
Parameters Used For Alloy Design The D F Orbital Energy Levels
Sparknotes Molecular Orbitals Molecular Orbital Theory
Fluorine Orbital Diagram Daytonva150
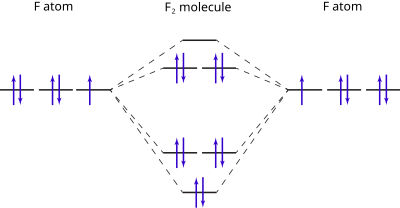 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
Mo Diagrams For Linear And Bent Molecules
Molecular Orbitals In Hydrogen Fluoride
F Block Orbital Diagram Wiring Diagram
 Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack
Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack
What Is An Orbital Diagram Orbital Diagram For Fluorine Awesome 0d
Molecular Orbitals In Fluorine
Diagram F Orbital Diagram 5 Linear F Orbital Diagram
Orbital Diagram For Fluorine Beautiful 6 4 Electronic Structure Of
Orbital Diagram For Fluorine Air American Samoa
 Electron Configuration Mapping The Electrons Electron Configuration
Electron Configuration Mapping The Electrons Electron Configuration
How Do You Draw S P D F Orbitals Socratic
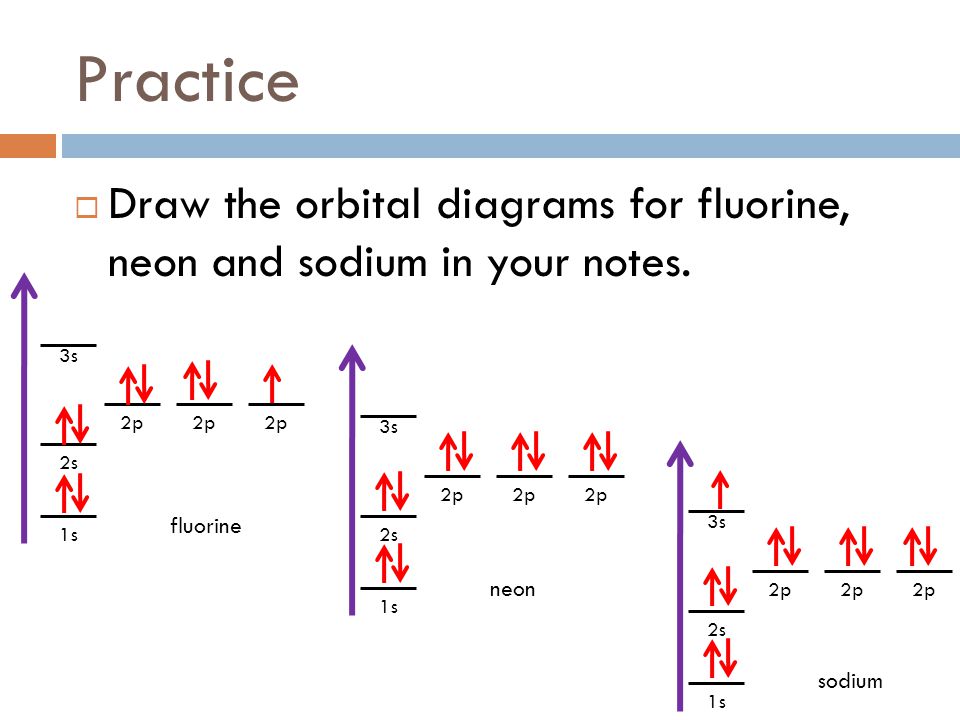 Fall 2011 Week 8 Electrons Ppt Download
Fall 2011 Week 8 Electrons Ppt Download
What Is An Orbital Diagram Orbital Diagram For Fluorine Awesome 0d
 Metal To Metal Multiple Bonds In Ordered Assemblies Pnas
Metal To Metal Multiple Bonds In Ordered Assemblies Pnas
 Orbital Diagram Of The Unrestricted B3lyp Calculation On The Lowest
Orbital Diagram Of The Unrestricted B3lyp Calculation On The Lowest
Diagram F Orbital Diagram The Below Violates Rule Because F

0 Response to "Orbital Diagram For F"
Post a Comment