Energy Diagram For Endothermic Reaction
It is difficult to measure the absolute energy of a substance but the change in energy during chemical reactions can be easily measured. Energy profile diagrams for endothermic and exothermic reactions.
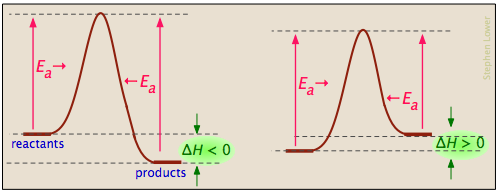 Basics Of Reaction Profiles Chemistry Libretexts
Basics Of Reaction Profiles Chemistry Libretexts
A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small.
Energy diagram for endothermic reaction. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. At the top of the curve the bonds in the reactants have been broken. Energy must be input in order to raise the particles up to the higher energy level.
Energy level diagram for an exothermic reaction is shown below. An energy level diagram shows whether a reaction is exothermic or endothermic. In other words the products are less stable than the reactants.
Energy flow endothermic reactions the reactants have less potential energy than do the products. Energy is being put in to break bonds in the reactants. Energy reactants products exothermic reactions the reactants have more potential energy than the products have.
Unfortunately for many reactions the real shapes of the energy profiles are slightly different from these and the rest of this page explores some simple differences. This energy diagram shows an exothermic reaction one in which energy is given off. This energy is given the symbol h and is different for different substances.
In the energy diagram for an endothermic reaction the energy of the products would be higher than that of the reactants. Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy diagrams for endothermic and exothermic reactions.
It also shows the effect of a catalyst on the forward and reverse activation energy. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. If you had an endothermic reaction a simple energy profile for a non catalysed reaction would look like this.
This is because there will be an observable energy exchange between the chemicals and the surroundings. Going from the reactants to the top of the curve we are going up the energy axis of the graph. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases.
More on pe diagrams. The amount of energy put in to break these bonds is called. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below.
 C J S B S Class Chemisty Energy Diagrams
C J S B S Class Chemisty Energy Diagrams
 Energy Diagram Of An Exothermic Reaction The Total Energy Is
Energy Diagram Of An Exothermic Reaction The Total Energy Is
 How To Read Potential Energy Diagrams
How To Read Potential Energy Diagrams
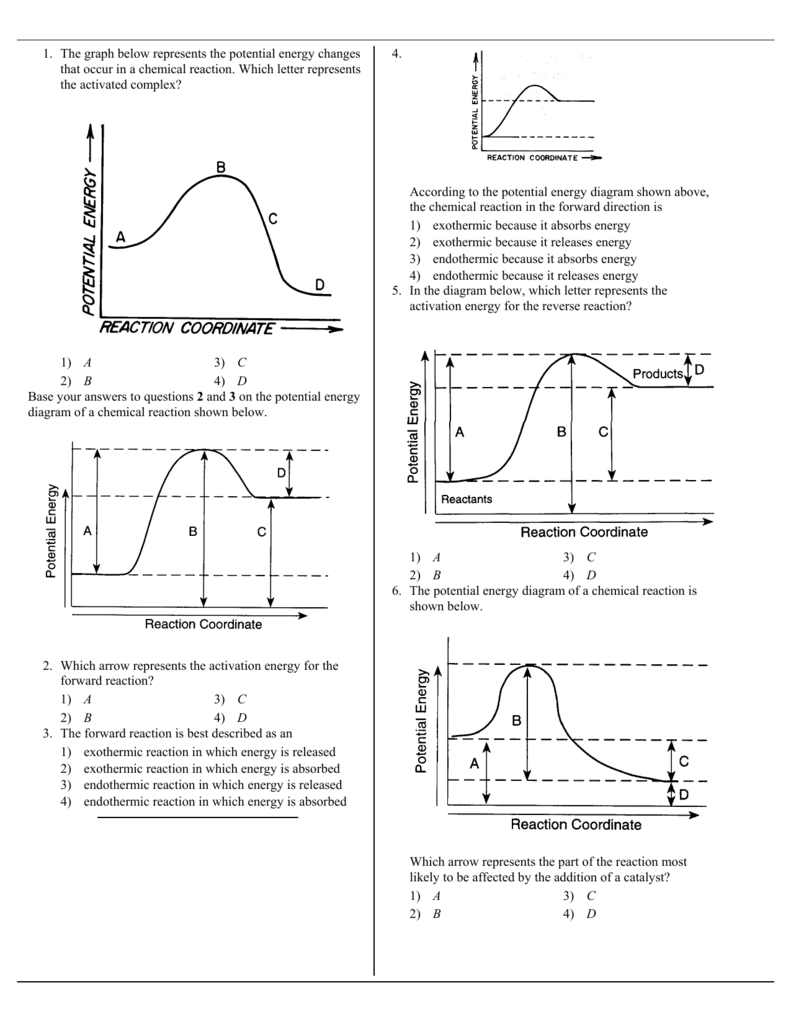 1 The Graph Below Represents The Potential Energy
1 The Graph Below Represents The Potential Energy
Exothermic And Endothermic Changes
 Activation Energy And The Activated Complex Energy And Chemical
Activation Energy And The Activated Complex Energy And Chemical
Energy Diagram For Exothermic Reaction Kejomoro Fresh Ideas
 Dublin Schools Lesson Bond Energy
Dublin Schools Lesson Bond Energy
Potential Energy Diagram Worksheet
 Solved Draw An Energy Diagram For An Endothermic Reaction
Solved Draw An Energy Diagram For An Endothermic Reaction

 Energy Level Diagram For An Endothermic Reaction Ap Chemistry
Energy Level Diagram For An Endothermic Reaction Ap Chemistry
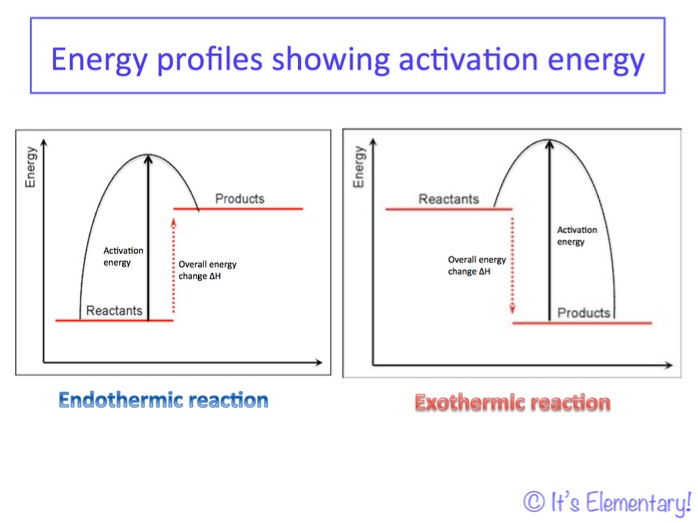 2016 Aqa Gcse Chemistry Unit 5 Lesson 2 Energy Profiles For
2016 Aqa Gcse Chemistry Unit 5 Lesson 2 Energy Profiles For
 How Does The Energy Level Diagram Show This Reaction Is Exothermic
How Does The Energy Level Diagram Show This Reaction Is Exothermic
Energy Diagram Endothermic And Exothermic Reaction Amazing Test
Energy Diagram Endothermic And Exothermic Reaction Luxury Enthalpy
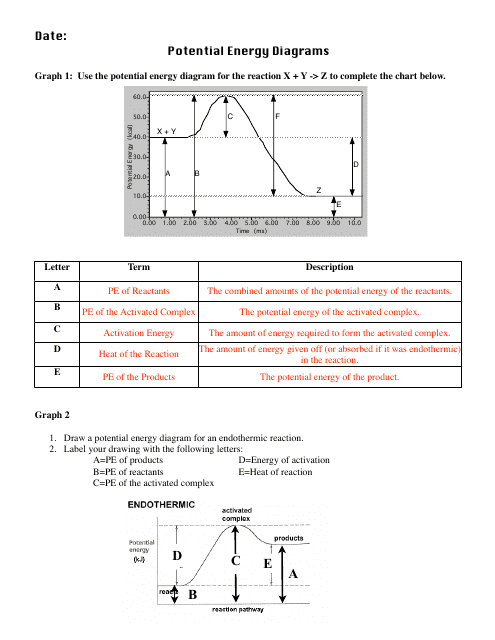 Potential Energy Diagrams Worksheet With Answers Download Printable
Potential Energy Diagrams Worksheet With Answers Download Printable
 4 14 Represent Exothermic And Endothermic Reactions On A Simple
4 14 Represent Exothermic And Endothermic Reactions On A Simple
 Mechanism Of Reaction And Catalysis Rate And Extent Of Reaction
Mechanism Of Reaction And Catalysis Rate And Extent Of Reaction
 Activation Energy And The Activated Complex Energy And Chemical
Activation Energy And The Activated Complex Energy And Chemical
 Endothermic Vs Exothermic Reaction Graphs Youtube
Endothermic Vs Exothermic Reaction Graphs Youtube
Reaction Energy Diagram For A One Step Exothermic Reaction

0 Response to "Energy Diagram For Endothermic Reaction"
Post a Comment