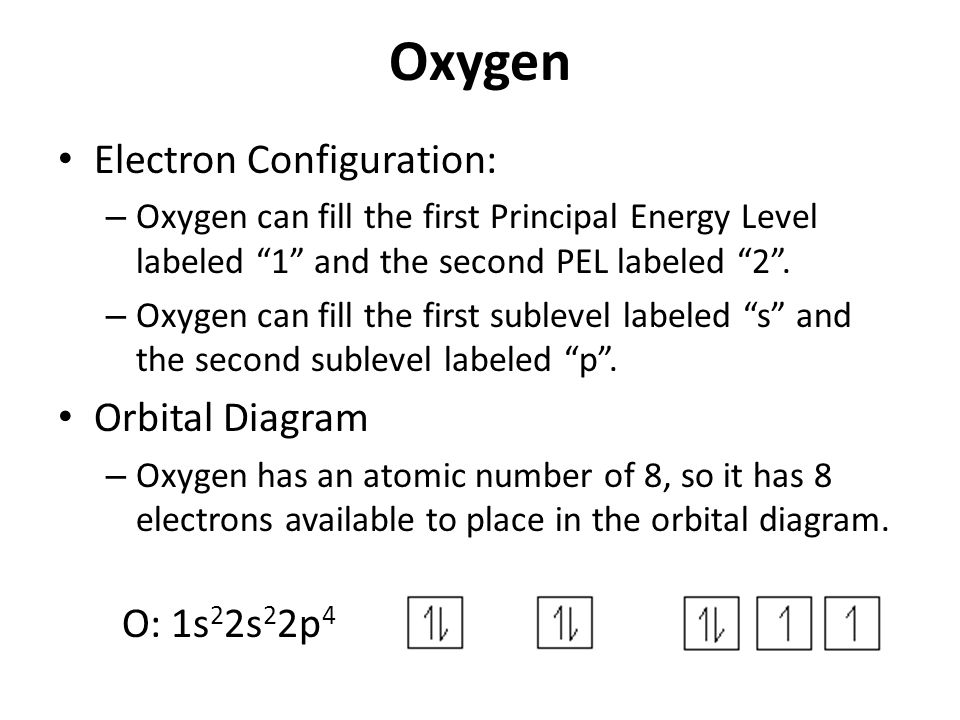
Use The Orbital Diagram For Oxygen To Write Quantum Numbers For The 4th Electron Of The O Atom
The first three quantum numbers of an electron are n1. Write the electron configuration for phosphorus and draw the orbital diagram.
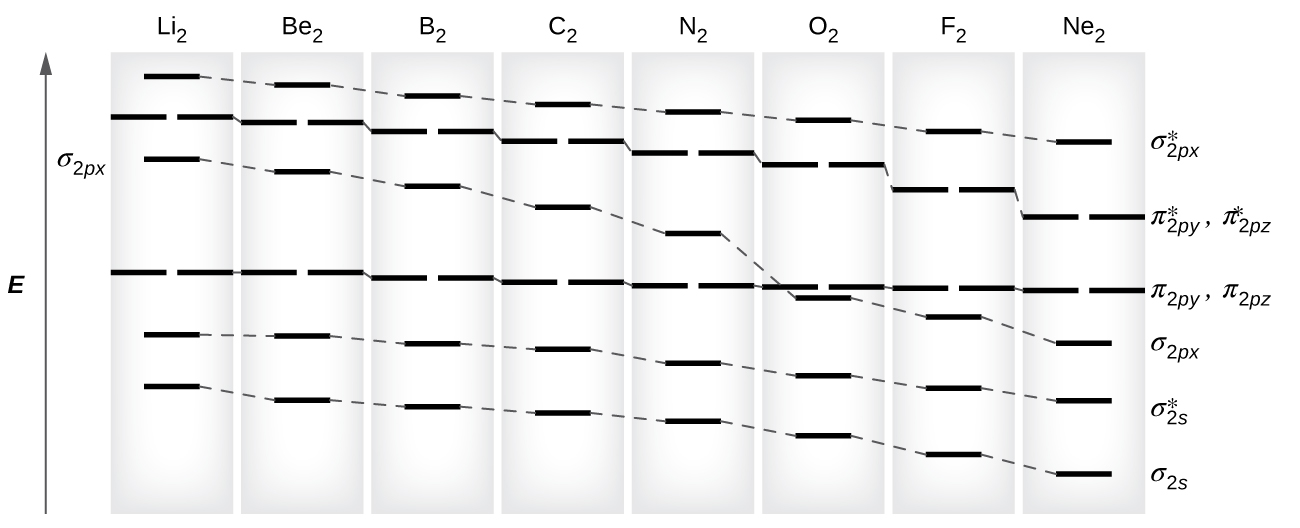
Molecular Orbital Theory Chemistry Encyclopedia Structure
In addition to listing the principle quantum number n and the subshell ell the orbital diagram shows all the different orientations and the spin of every electron.
Use the orbital diagram for oxygen to write quantum numbers for the 4th electron of the o atom. The diagram shows the number of subshell by using boxes or lines for electrons use three for p orbitals five for d orbitals and 7 for f orbitals. N 1 2 3. The orbital filling diagram of lithium.
The remaining four electrons will go in the 2p orbital. 1 charge means lost one electron write the normal electron configuration for na then remove an electron from the last orbital. The electron configuration for phosphorus.
The electron configuration of lithium is 1s²2s¹. This number means that oxygen has 8 protons in its nucleus and 8 electrons. All orbitals that have the same value of n are said to be in the same shell level.
Oxygen is the eighth element with a total of 8 electrons. A step by step description of how to write the electron configuration for oxygen o. The pauli exclusion principle states that no two electrons in an atom can have the same four quantum numbers electrons in the same orbital must.
N represents the energy level l is associated with the sublevel ml represents the orbital and ms is the electron spin. Electron configuration and orbital diagrams part 1. In order to write the o electron configuration we first need to know the number of electrons for the o atom.
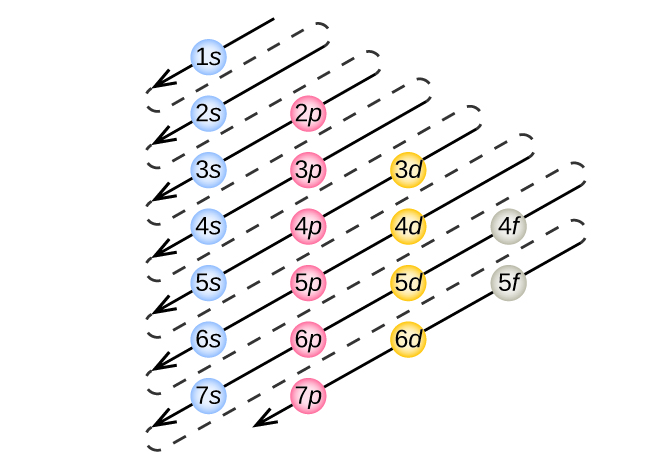
When writing the electron configuration for an atom orbitals are filled in order of increasing atomic number. How to write the electron configuration for oxygen. This means that there are two electrons in the 1s orbital and one electron in the higher energy 2s orbital.
In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Principal quantum number n. You can represent electrons as arrows.
For news about the other quantum numbers you really should check the appropriate tutorial. So you put 8 electrons into your energy level diagram. Since 1s can only hold two electrons the next 2 electrons for o go in the 2s orbital.
Specifies the energy of an electron and the size of the orbital the distance from the nucleus of the peak in a radial probability distribution plot. If two electrons end up in the same orbital one arrow faces up and the other faces down. Orbitals are built up from atom to atom.
It discusses the 4 quantum numbers n l ml and ms.
 6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
 Pdf Atomic Structure Basic Concepts Of Chemistry
Pdf Atomic Structure Basic Concepts Of Chemistry
 Quantum Numbers Atomic Orbitals And Electron Configurations
Quantum Numbers Atomic Orbitals And Electron Configurations
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
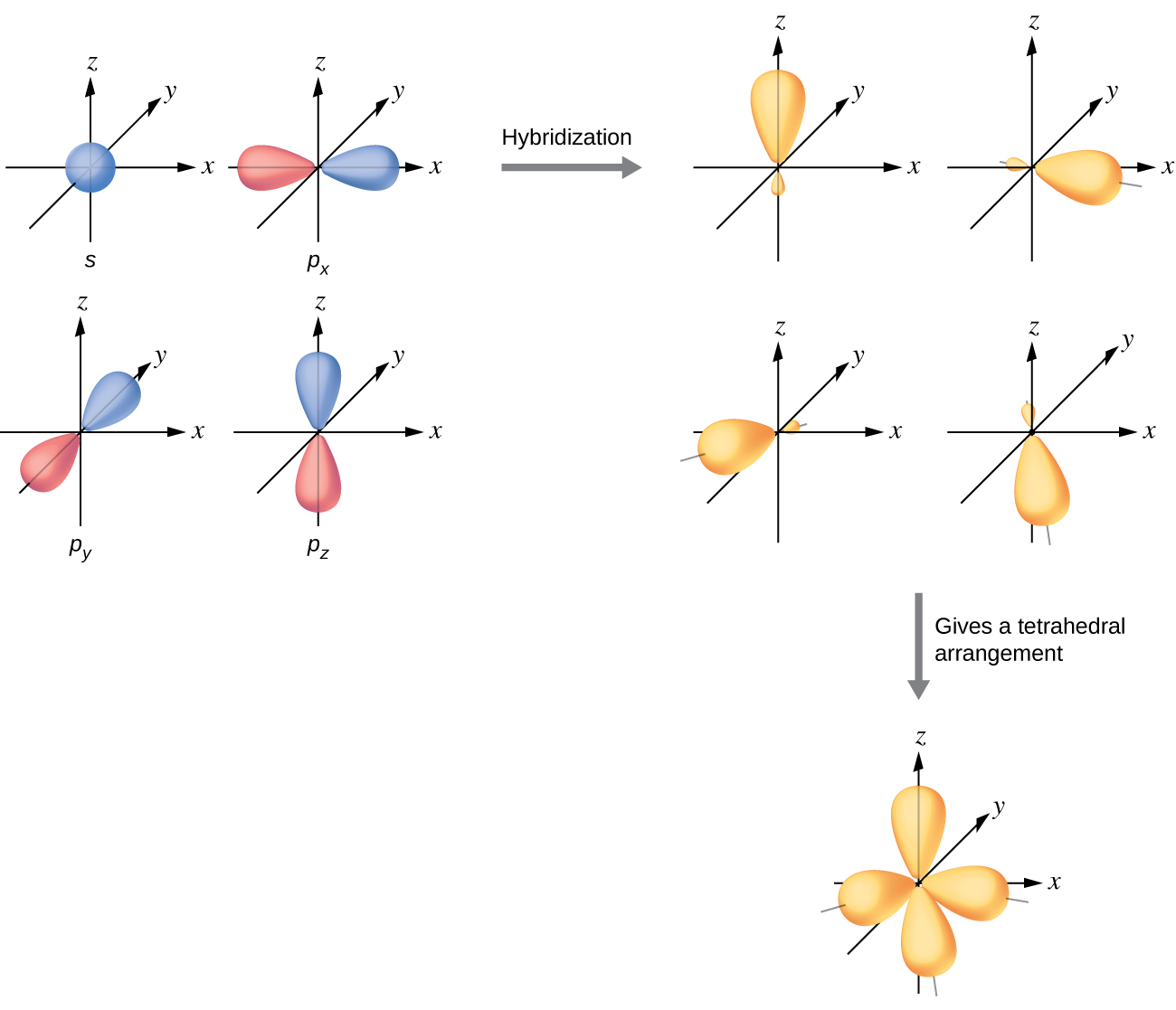 8 2 Hybrid Atomic Orbitals Chemistry
8 2 Hybrid Atomic Orbitals Chemistry
 Electron Configuration And Orbital Diagrams Ppt Video Online Download
Electron Configuration And Orbital Diagrams Ppt Video Online Download
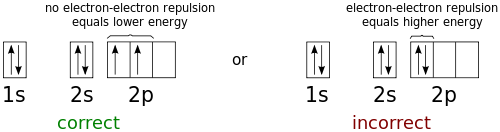 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
Quantum Numbers And Electron Configurations
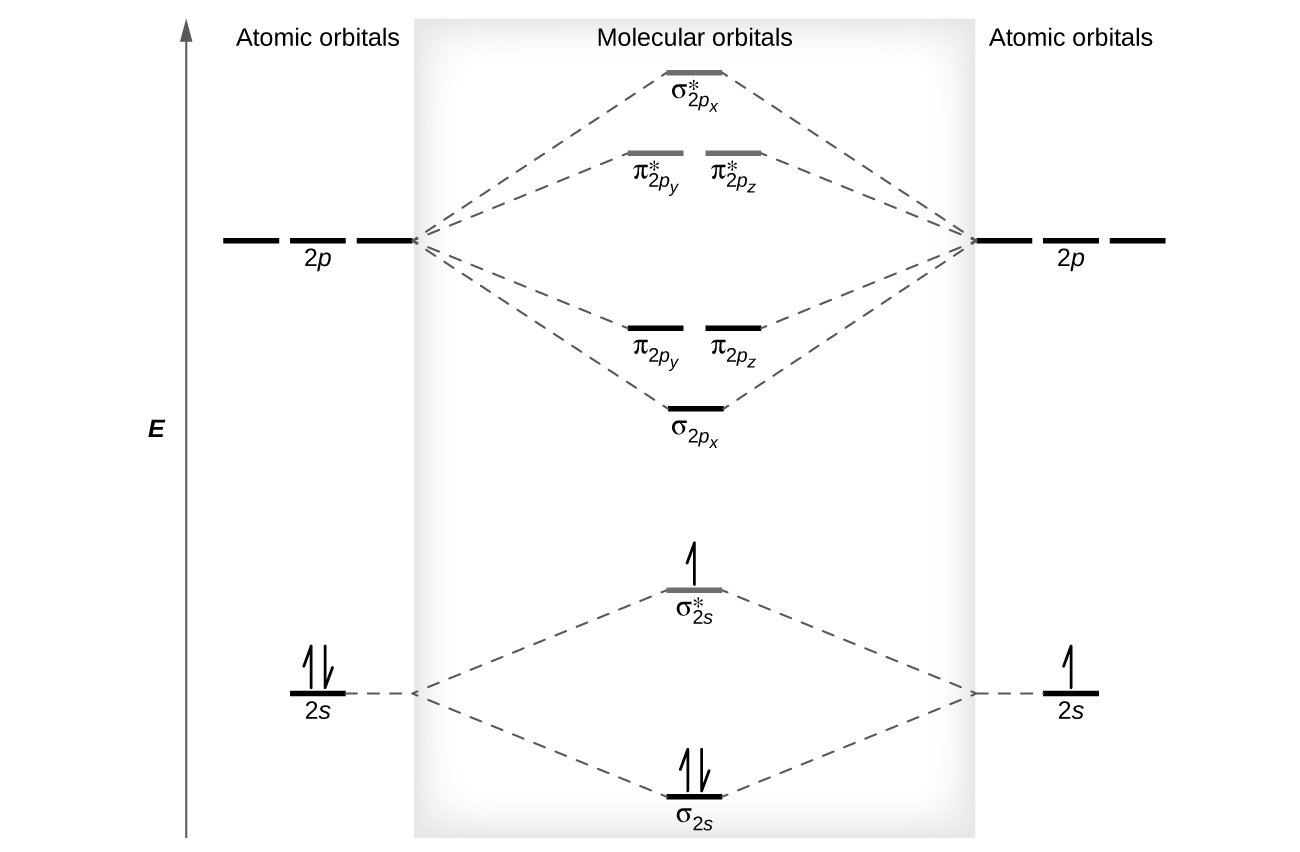 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
 What Is The Correct Set Of Quantum Numbers N L Ml Ms For The
What Is The Correct Set Of Quantum Numbers N L Ml Ms For The
 What Are Inner Shell Electrons Sciencing
What Are Inner Shell Electrons Sciencing
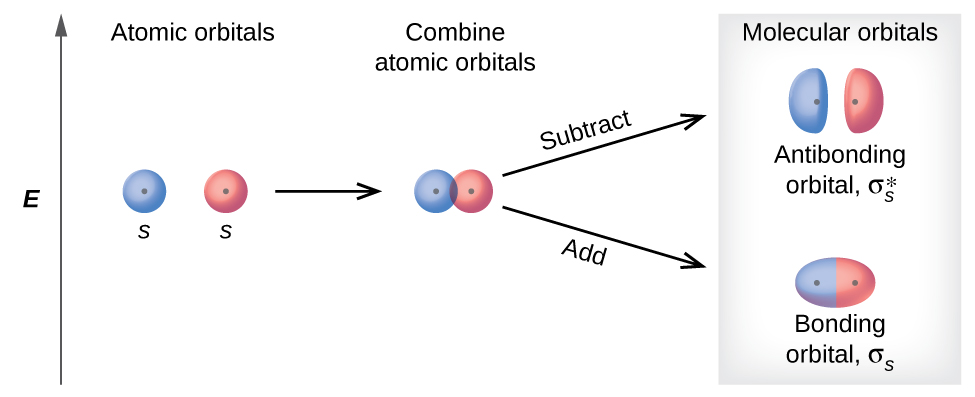 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
:max_bytes(150000):strip_icc()/aufbauexample-56a129555f9b58b7d0bc9f48.jpg) Electronic Structure And The Aufbau Principle
Electronic Structure And The Aufbau Principle
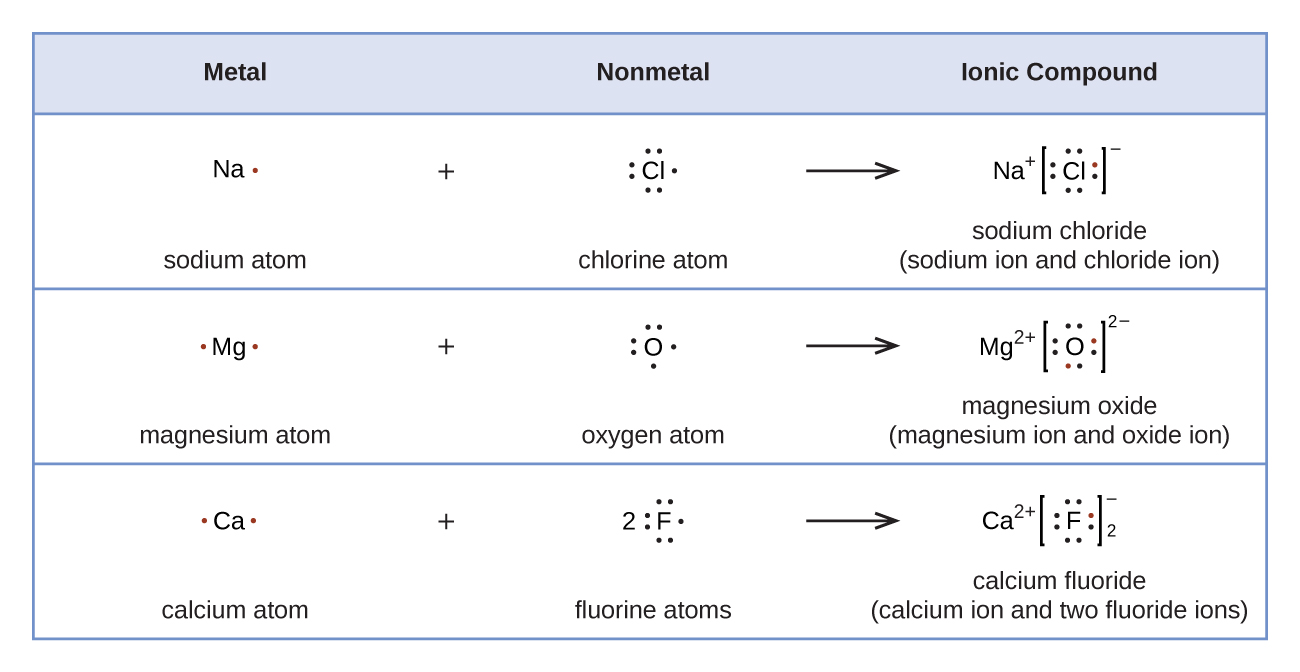 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
 Four Quantum Numbers Principal Angular Momentum Magnetic Spin
Four Quantum Numbers Principal Angular Momentum Magnetic Spin
 Azimuthal Quantum Number Wikipedia
Azimuthal Quantum Number Wikipedia
 Azimuthal Quantum Number Wikipedia
Azimuthal Quantum Number Wikipedia

0 Response to "Use The Orbital Diagram For Oxygen To Write Quantum Numbers For The 4th Electron Of The O Atom"
Post a Comment