Electron Dot Diagram For Boron
3 are in the valence shell. It was discovered as an unstable gas and only in 2009 found to be a stable ligand combining with transition metals in the same way as carbon monoxide.
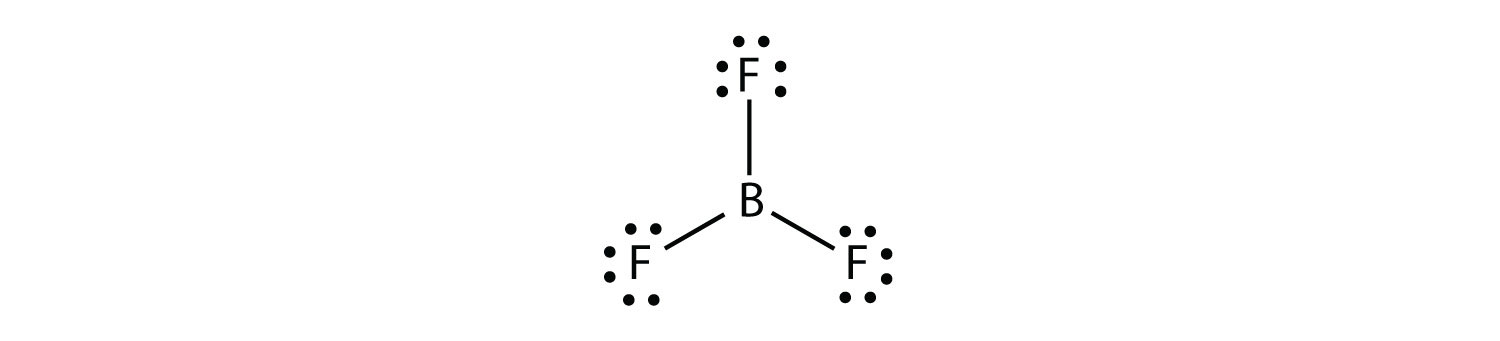
Thus the structure of bf 3 with single bonds and 6 valence electrons around the central boron is the most likely structure.
Electron dot diagram for boron. In drawing lewis structures the dots presented on the atoms are the valence electrons present in the valence shell of that particular atom. Threedots belong in the electron dot diagram of a boron. The next atom is boron.
The next atom is boron. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. Boron is the fifth element with a total of 5 electrons.
Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom. The third electron will go on another side of the symbol. More than an octet most common example of exceptions to the octet rule.
Boron is in group 13 sometimes called group iii or 3a. Here is a table showing the first 20 atoms by atomic number. Its valence electron shell is 2s 2 2p 1 so it has three valence electrons.
Lewis electron dot diagrams for ions have less for cations or more for anions dots than the corresponding atom. Bf 3 reacts strongly with compounds which have an unshared pair of electrons which can be used to form a bond with the boron. Its valence electron shell is 2s 2 2p 1 so it has three valence electrons.
The third electron will go on another side of the symbol. It is a useful lewis acid and a versatile building block for other boron compounds. Boron monofluoride or fluoroborylene is a chemical compound with formula bf one atom of boron and one of fluorine.
After that i draw the lewis dot structure for boron b. Boron trifluoride is the inorganic compound with the formula bf 3. Since it is in group 3 it will have 3 valence electrons.
Since 1s can only hold two electrons the next 2 electrons for b goes in the 2s orbital. A chemistry book perhaps. Boron has 5 electrons.
So boron can use those electrons to bond with three other atoms. This pungent colourless toxic gas forms white fumes in moist air.
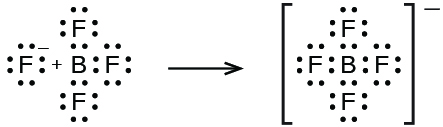 18 3 Structure And General Properties Of The Metalloids Chemistry
18 3 Structure And General Properties Of The Metalloids Chemistry
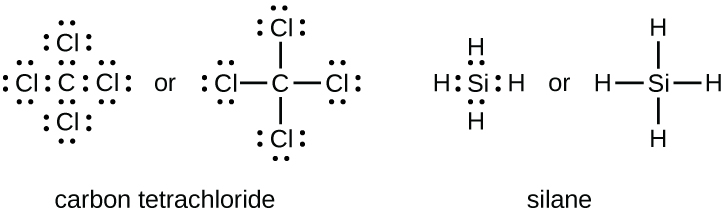 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
Boron Trioxide B2o3 Chemspider
 Why Is Boron Trifluoride Written In Two Ways In The Lewis Dot
Why Is Boron Trifluoride Written In Two Ways In The Lewis Dot
 Solved Draw A Lewis Dot Structure For Nabh4 And Indicate The
Solved Draw A Lewis Dot Structure For Nabh4 And Indicate The

 Dot And Cross Model Of Sodium Borohydride Or Lithium Aluminium
Dot And Cross Model Of Sodium Borohydride Or Lithium Aluminium
Lewis Dot Diagram Boron Fabulous Lewis Dot Structure For Beryllium
 Lewis Dot Structure For Boron Atom B Youtube
Lewis Dot Structure For Boron Atom B Youtube
 1 Modern Atomic Theory 2 In The Rutherford Model Electrons Traveled
1 Modern Atomic Theory 2 In The Rutherford Model Electrons Traveled
Regents Chemistry Exam Explanations January 2009
 Why Is Bf3 Written In Two Ways Quora
Why Is Bf3 Written In Two Ways Quora
Violations Of The Octet Rule Chemistry Libretexts
 Xenon Dot Diagram 20 Artatec Automobile De
Xenon Dot Diagram 20 Artatec Automobile De
 Install Electron Dot Diagram For Hydrogen Chloride Toyskids Co
Install Electron Dot Diagram For Hydrogen Chloride Toyskids Co
Lewis Dot Diagram For Transition Metals Lovely How To Draw A Lewis
Boron Electron Dot Diagram File Lewis Dot Tlg Wikimedia Mons Air
Dot Structures I Single Bonds Video Khan Academy
 Boron Carbide Structure Download Scientific Diagram
Boron Carbide Structure Download Scientific Diagram
Violations Of The Octet Rule Chemistry Libretexts
 Why Is Boron Trifluoride Written In Two Ways In The Lewis Dot
Why Is Boron Trifluoride Written In Two Ways In The Lewis Dot

0 Response to "Electron Dot Diagram For Boron"
Post a Comment