Label The Following Reaction Energy Diagram For A Catalyzed And An Uncatalyzed Process
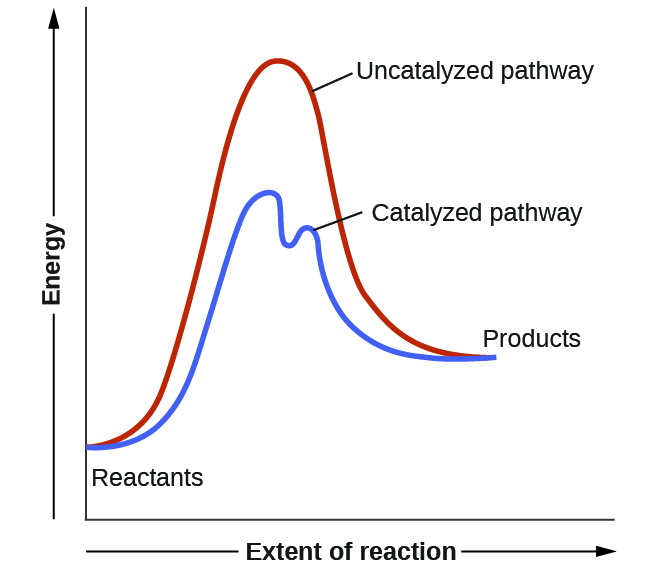
Sometimes a teacher finds it necessary to ask questions about pe diagrams that involve actual potential energy values. Lower the free energy of activation to a value below the free energy of the reactants and products.
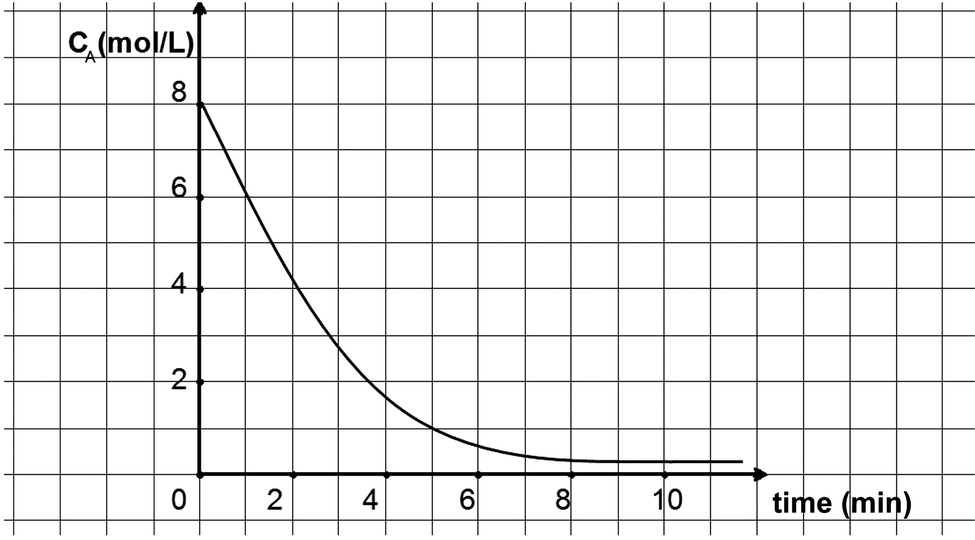 Investigating High School Chemical Kinetics The Greek Chemistry
Investigating High School Chemical Kinetics The Greek Chemistry
For the chemical reaction system described by the diagram below which statemen.

Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. The activation energy of a reaction is 75 kjmol and the change in enthalpy i. Label your drawing with the following letters. A potential energy diagram plots the change in potential energy that occurs during a chemical reaction.
Label the energy diagram and answer the question that follows. Draw and label a reaction coordinate diagram for an uncatalyzed reaction s p and the same reaction catalyzed by an enzyme e. Raise the free energy of activation to a value above the free energy of the reactants and products.
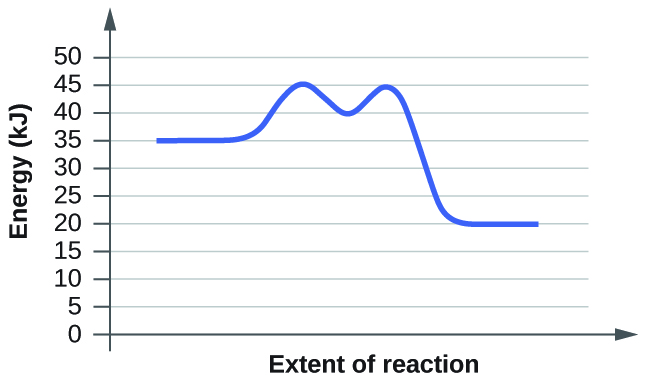
For the chemical reaction system described by the diagram below which stateme. Outline four targets of antimicrobial drugs. This means that less energy is required to convert reactant molecules to products.
Potential energy diagrams time ms 000 100 200 300 400 500 600 700 800 900 100 potential energy kcal 000. Activation energy is the energy that a reaction must overcome to convert a substrate to the product. Show transcribed image text enzymes are important molecules in biochemistry that catalyze reactions.
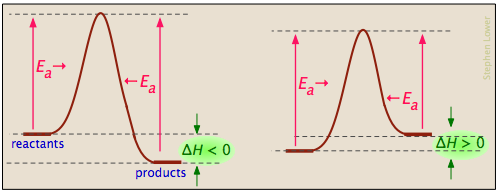
This first video takes you through all the basic parts of the pe diagram. Enzymes lower the activation energy of a chemical reaction just as all catalysts lower activation energy. Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction.
Draw a potential energy diagram for an endothermic reaction. Lower the free energy of activation to a value below that of the uncatalyzed reaction. It also shows the effect of a catalyst on the forward and reverse activation energy.
You will place all the labels in part a. Label the multi step reaction energy diagram below using the letters correspon. Show transcribed image text label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. Ape of products denergy of activation bpe of reactants eheat of reaction cpe of the activated complex date. Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
 Basics Of Reaction Profiles Chemistry Libretexts
Basics Of Reaction Profiles Chemistry Libretexts
Section 13 3 The Rate Of A Reaction
 Activation Energy And The Activated Complex Energy And Chemical
Activation Energy And The Activated Complex Energy And Chemical
 Energy Profile Chemistry Wikipedia
Energy Profile Chemistry Wikipedia
 Openstax General Chemistry Ch 12 Kinetics Top Hat
Openstax General Chemistry Ch 12 Kinetics Top Hat
 Gen Chem Discuss Chemical Equilibrium
Gen Chem Discuss Chemical Equilibrium
Sample Exercise 14 1 Calculating An Average Rate Of Reaction
 Label The Following Reaction Coordinate Di Clutch Prep
Label The Following Reaction Coordinate Di Clutch Prep
 Factors Affecting Reaction Rates Chemistry For Majors
Factors Affecting Reaction Rates Chemistry For Majors
What Is The Activation Energy For A Reverse Reaction Quora
 Answer Label The Energy Diagram For A Two Clutch Prep
Answer Label The Energy Diagram For A Two Clutch Prep
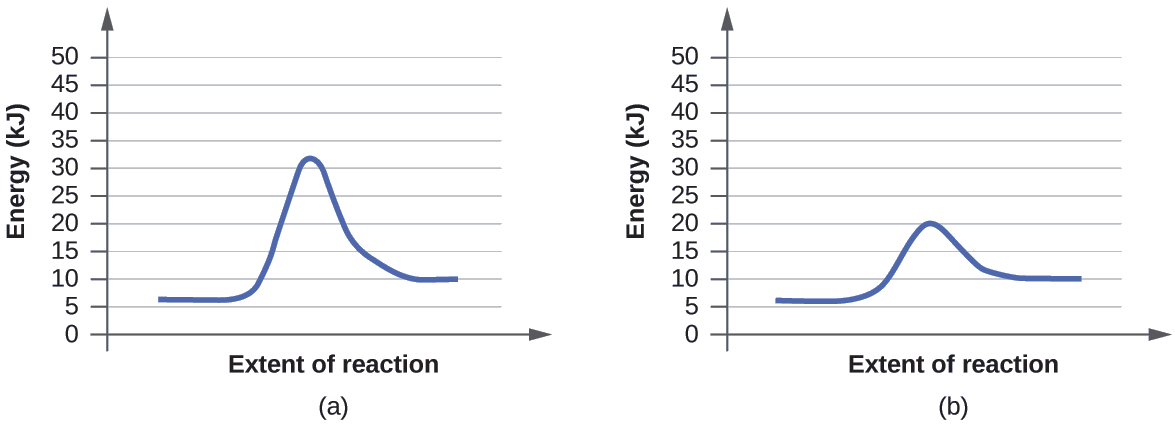 12 7 Catalysis Chemistry Libretexts
12 7 Catalysis Chemistry Libretexts
Energy And Enzymes Biology 1510 Biological Principles
 Answer Label The Energy Diagram For A Two Clutch Prep
Answer Label The Energy Diagram For A Two Clutch Prep
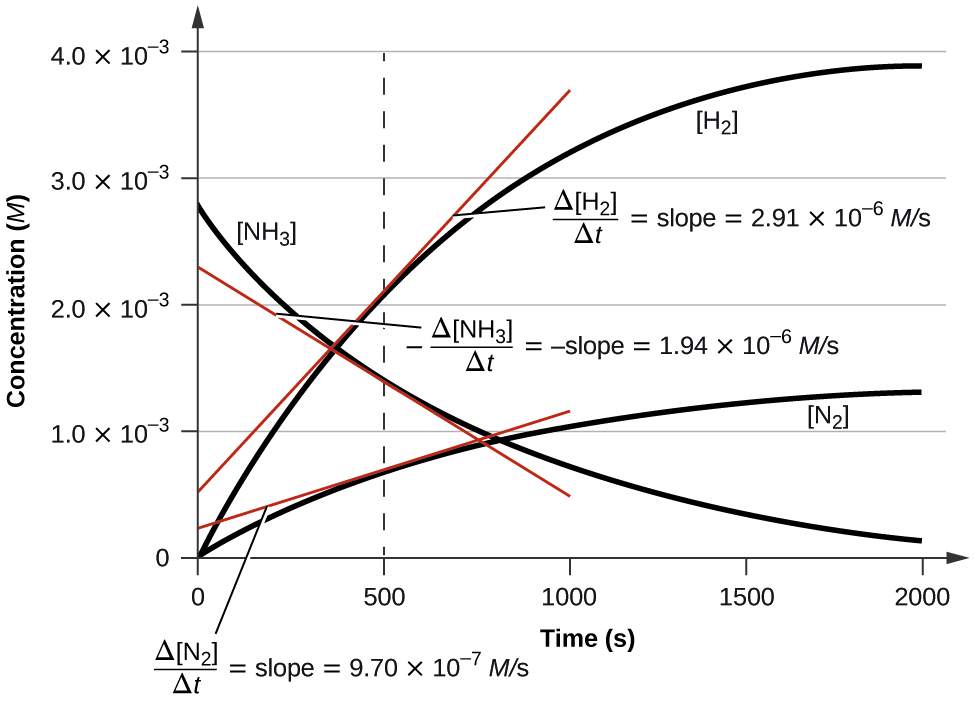 Openstax General Chemistry Ch 12 Kinetics Top Hat
Openstax General Chemistry Ch 12 Kinetics Top Hat
Potential Kinetic Free And Activation Energy Boundless Biology

0 Response to "Label The Following Reaction Energy Diagram For A Catalyzed And An Uncatalyzed Process"
Post a Comment