No Molecular Orbital Diagram
Molecular orbitals of no. Step 1 of 5 molecular orbitals are formed by linear combination of atomic orbitals.
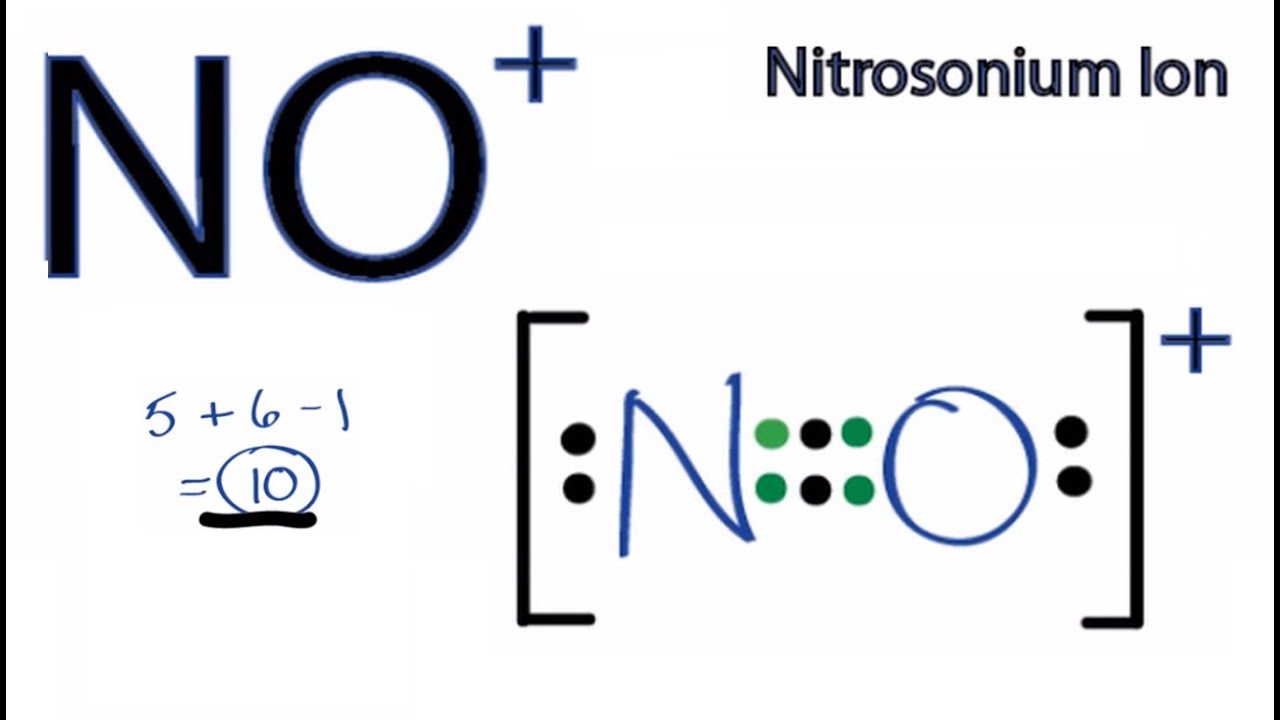 No Lewis Structure How To Draw The Lewis Structure For No
No Lewis Structure How To Draw The Lewis Structure For No
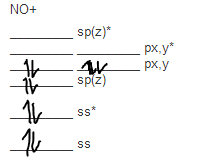
Note the odd electron is in a pi2p orbital.
No molecular orbital diagram. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. Molecular orbital diagram of no endorsed post by claudettecontr3i tue nov 15 2016 1000 pm i am not quiet sure but it seems that the book may have taken the electron off o because then it would have the same amount of electrons in the 2p orbital as nitrogen it looks neater. Compare the bond orders in these two ions.
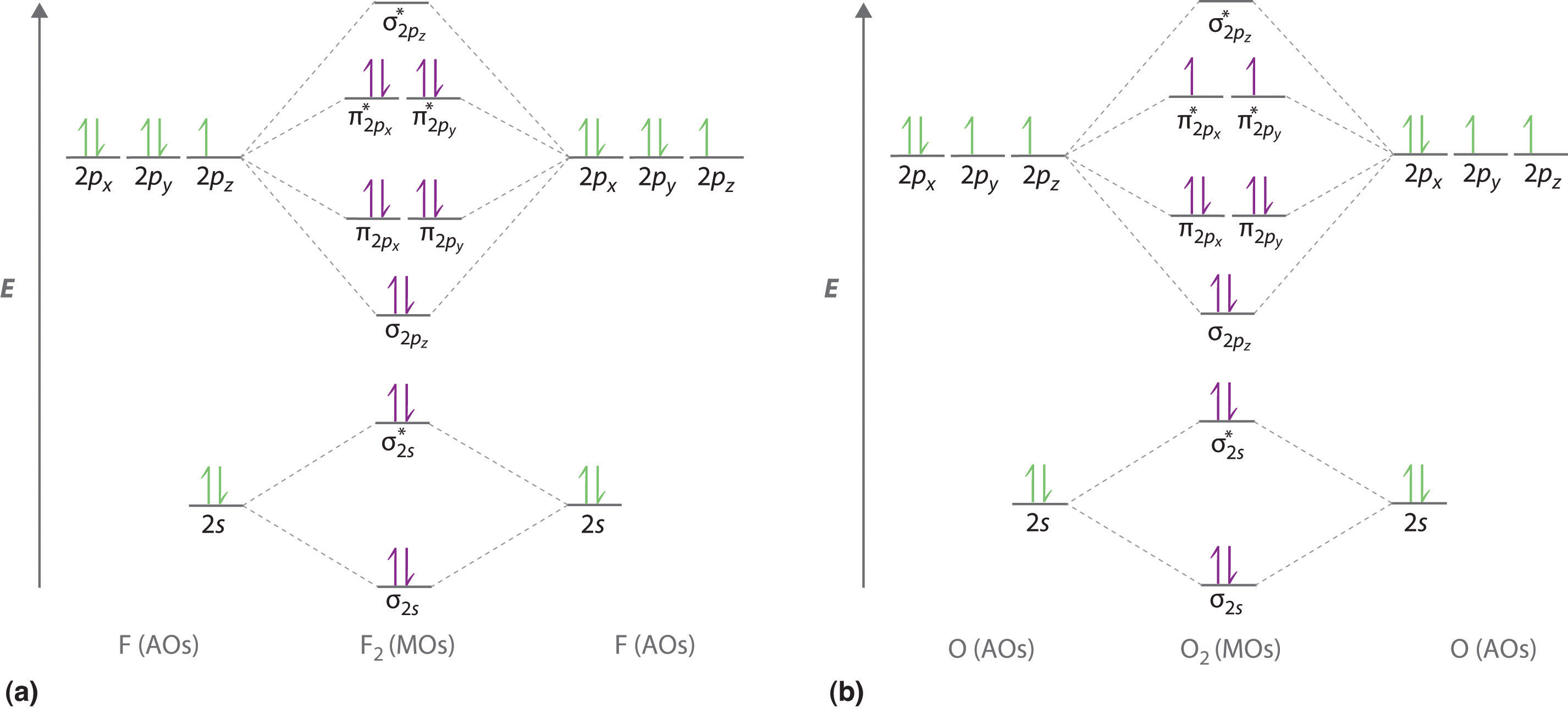
This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. Introductory molecular orbital theory. No bond order 3 shortest bond 106 pm no bond order 25 intermediate 115 pm no bond order 2 longest bond 127 pm two electrons in antibonding orbitals.
In chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule. In chemistry a molecular orbital mo is a mathematical function describing the wave like behavior of an electron in a molecule. The bonding orbitals are slightly more concentrated on o.
The course introduces the three key spectroscopic methods used by chemists and biochemists to analyse the molecular and electronic structure of atoms and molecules. O is more electronegative than n so its orbitals are slightly lower in energy and the bonding orbitals are slightly more concentrated on o. Computational study of the interaction between no no and no with h2o.
The bond order is 25 with one unpaired electron. Atomic orbitals and molecular orbitals of a molecule can be shown in a molecular orbital diagram. Now draw two more mo diagrams for no and no your no diagram will have one less valence electron and your no diagram will have one more valence electron.
Draw the molecular orbital diagrams for no and no. These are uvvisible infra red ir and nuclear magnetic resonance nmr spectroscopies. An honors general chemistry computational lab as implemented using three dimensional modeling software journal of chemical education ruddick parrill and petersen 2012 89 11 pp 13581363 abstract.
Molecular orbital diagrams of diatomic molecules introduction.
 Solved 1 Draw Rough Molecular Orbital Diagrams For The G
Solved 1 Draw Rough Molecular Orbital Diagrams For The G
Molecular Orbital Theory Mot Chemistry Study Material
 Electronic Configuration Where Does The 9th Electron Go In A N O
Electronic Configuration Where Does The 9th Electron Go In A N O
 Experiment 7 Experiment 7 Computational Modeling Of Molecular
Experiment 7 Experiment 7 Computational Modeling Of Molecular
 Chapter 6 5 Delocalized Bonding And Molecular Orbitals Chemistry
Chapter 6 5 Delocalized Bonding And Molecular Orbitals Chemistry
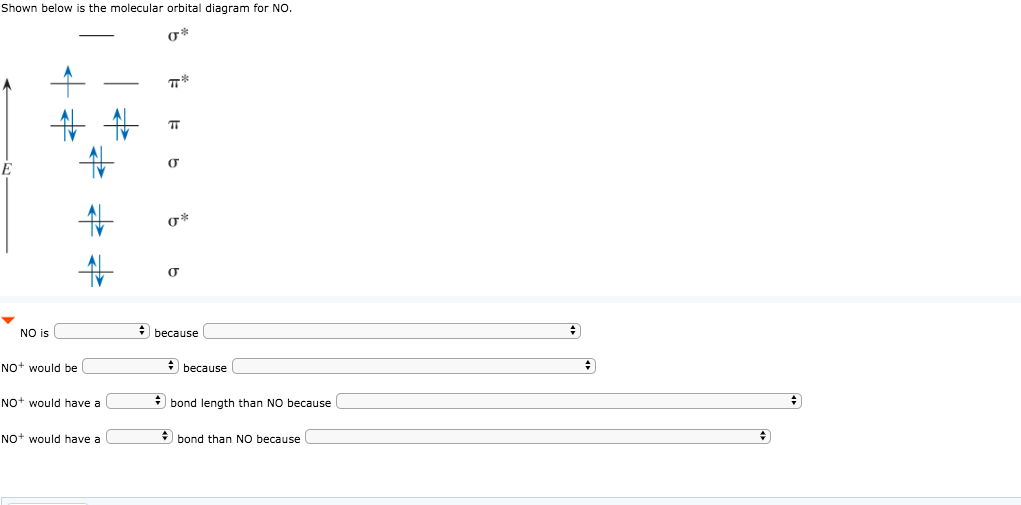 Solved Shown Below Is The Molecular Orbital Diagram For N
Solved Shown Below Is The Molecular Orbital Diagram For N
 The Coordination Chemistry Of Nitrosyl In Cyanoferrates An Exhibit
The Coordination Chemistry Of Nitrosyl In Cyanoferrates An Exhibit
Chem 2303 Supplementary Problems
 Pdf Lewis Acidity Of No And No2 As Measured By Their Affinity To
Pdf Lewis Acidity Of No And No2 As Measured By Their Affinity To
Estimating Local Bonding Antibonding Character Of Canonical
 Orbitals What Is The Origin Of The Differences Between The Mo
Orbitals What Is The Origin Of The Differences Between The Mo
 Solved The Orbital Energy Ladder Molecular Orbital Energ
Solved The Orbital Energy Ladder Molecular Orbital Energ
 Mo Diagram Of No 7 2 Stromoeko De
Mo Diagram Of No 7 2 Stromoeko De
 What Is The Bond Order Of Co Quora
What Is The Bond Order Of Co Quora
Chemistry 424 Organometallic Chemistry
 Chapter 6 5 Delocalized Bonding And Molecular Orbitals Chemistry
Chapter 6 5 Delocalized Bonding And Molecular Orbitals Chemistry
 Basic Sites In Zeolites Followed By Ir Studies Of No Sciencedirect
Basic Sites In Zeolites Followed By Ir Studies Of No Sciencedirect
 Energy Molecular Orbital Diagram For Nitrogen Monoxide The
Energy Molecular Orbital Diagram For Nitrogen Monoxide The
 Chapter 10 Molecular Geometry And Chemical Bonding Theory Ppt
Chapter 10 Molecular Geometry And Chemical Bonding Theory Ppt
0 Response to "No Molecular Orbital Diagram"
Post a Comment