The Line Connecting The Triple Point And The Critical Point On A Phase Diagram Represents
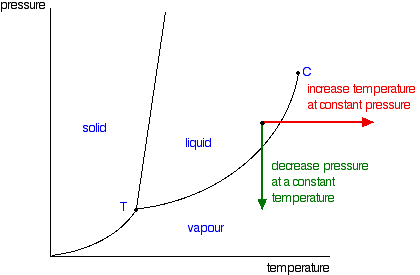
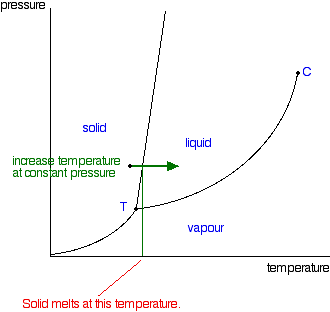
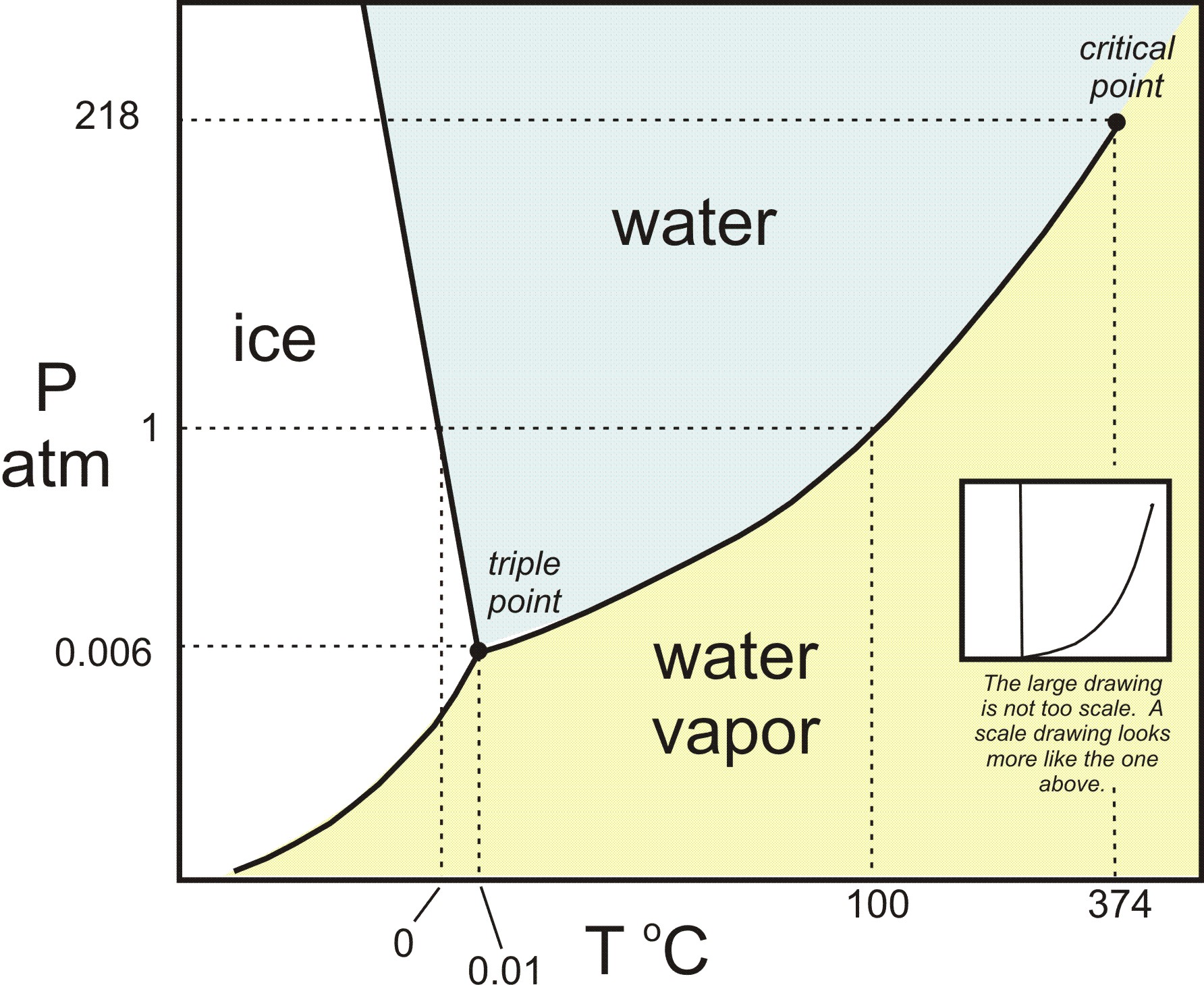
The temperature and pressure combinations above which only a supercritical fluid can exist the temperature and pressure combinations at which the liquid and solid states are equally stable and at equilibrium. At the critical point of a substance only gaseous and liquid phases can exist in equilibrium.
Pressure Temperature Diagram P T Diagram Fundamentals Of Fluid
The line connecting the triple point and the critical point on a phase diagram represents a.
The line connecting the triple point and the critical point on a phase diagram represents. The line connecting the triple point and the critical point on a phase diagram represents. What is a phase diagram. B a phase diagram is simply a map of the phase of a substance as a function of volume.
The line connecting the triple point and the critical point on a phase diagram represents. These lines show where two phases are in equilibrium. There are three solid lines on the phase diagram that are called the phase equilibrium lines.
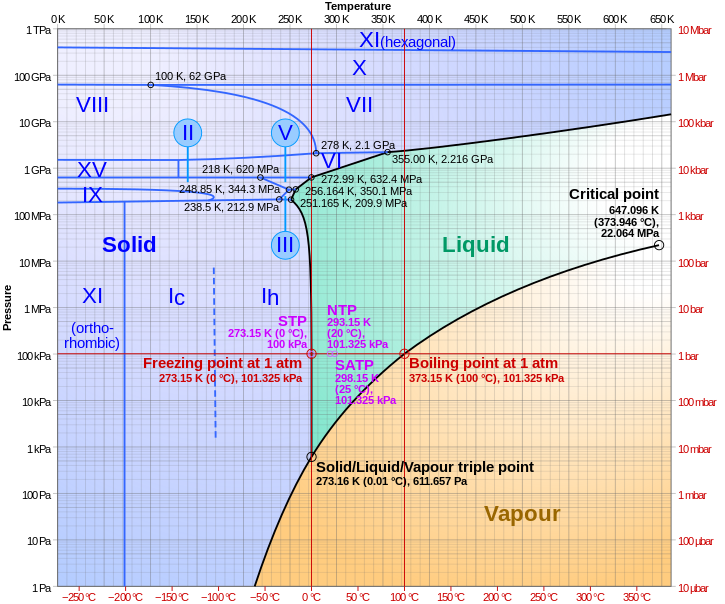
The line connecting the triple point and the critical point on a phase diagram represents. At critical point enthalpy entropy specific volume of saturated liquid and saturated vapour are equal. The temperatures and pressures above which only a supercritical fluid can exist c.
The triple point is the temperature and pressure at which the three phases of a substance can coexist. The critical point of a substance lies at the endpoint of the phase equilibrium curve whereas the triple point is the point where the three equilibrium curves meet. The critical point is the temperature and pressure above which a substance cannot exist as a liquid.
A a phase diagram is simply a map of the phase of a substance as a function of volume on the y axis and pressure on the x axis. At triple point all three phases solid liquid and gas are in equilibrium means these cant be separated at triple point. The temperatures and pressures at which the solid and gas states are equally stable and at equilibrium b.
B the temperatures and pressures at which the solid and gas states are equally stable and at equilibrium c the temperatures and pressures at which the liquid and solid states are equally stable and at equilibrium d the temperatures and pressures at which the liquid and gas states are equally stable and at equilibrium. The critical point on the phase diagram shows where the gas and liquid states of a liquid are identical and the substance is in one phase. The critical point is the critical pressure for turning a liquid into a solid.
The line connecting the triple point and the critical point on a phase diagram represents.
 Thermodynamics Ebook Property Diagrams
Thermodynamics Ebook Property Diagrams
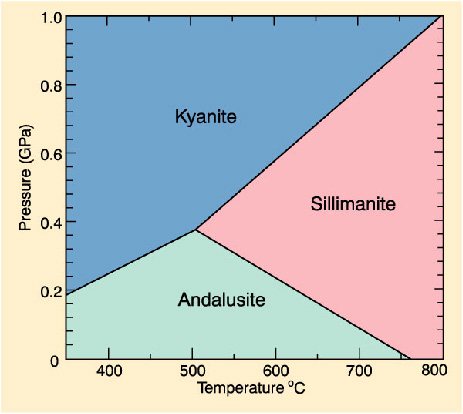
 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
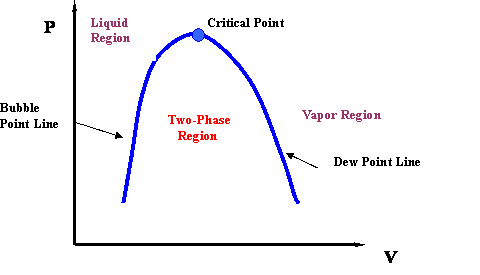 Pv Diagram For Pure Systems Png 520 Phase Behavior Of Natural Gas
Pv Diagram For Pure Systems Png 520 Phase Behavior Of Natural Gas
Thermodynamics Properties Of Pure Substances Pure Substance A
 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
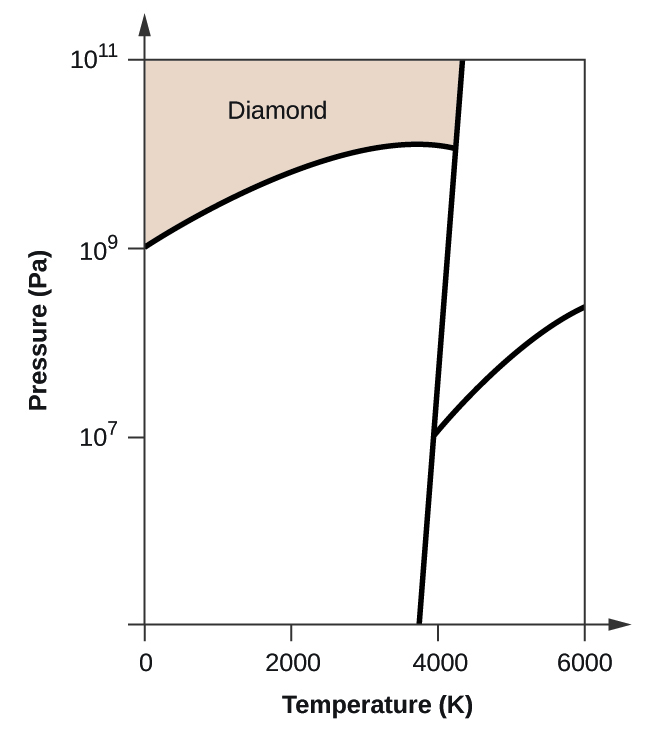
 Sublimation Phase Transition Wikipedia
Sublimation Phase Transition Wikipedia
 Phase Diagram Physics Britannica Com
Phase Diagram Physics Britannica Com
Thermodynamics Properties Of Pure Substances Pure Substance A
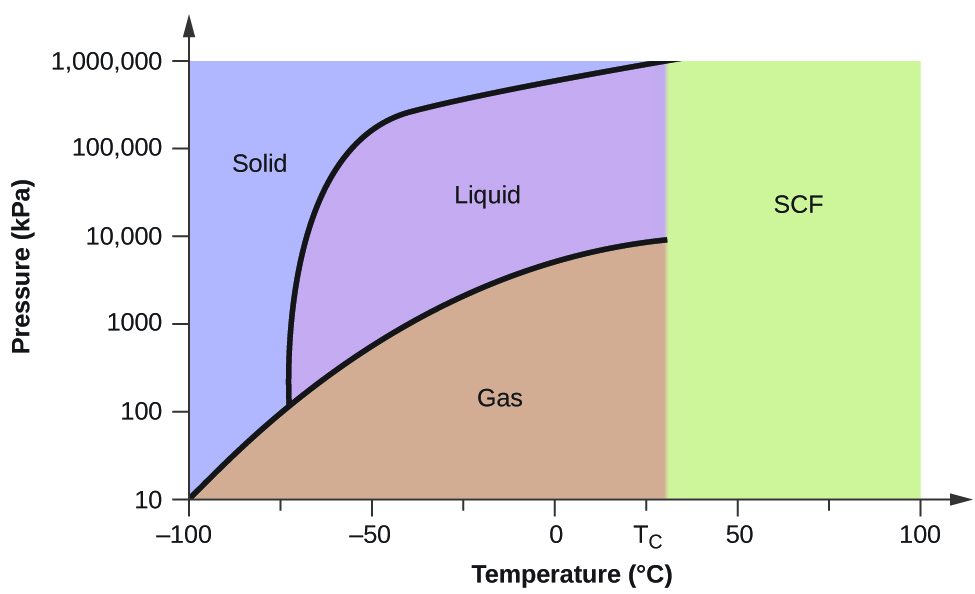
 Phase Diagrams An Overview Sciencedirect Topics
Phase Diagrams An Overview Sciencedirect Topics
 Phase Diagrams An Overview Sciencedirect Topics
Phase Diagrams An Overview Sciencedirect Topics
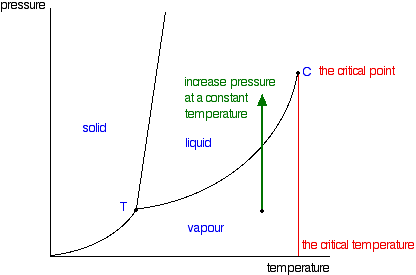 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
 Thermodynamics Ebook Property Diagrams
Thermodynamics Ebook Property Diagrams
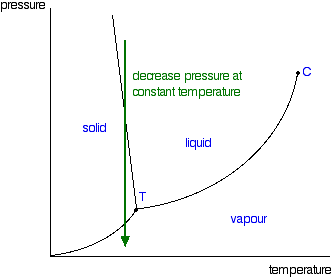 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
 Thermodynamics Ebook Property Diagrams
Thermodynamics Ebook Property Diagrams

0 Response to "The Line Connecting The Triple Point And The Critical Point On A Phase Diagram Represents"
Post a Comment