Molecular Orbital Diagram Of Benzene
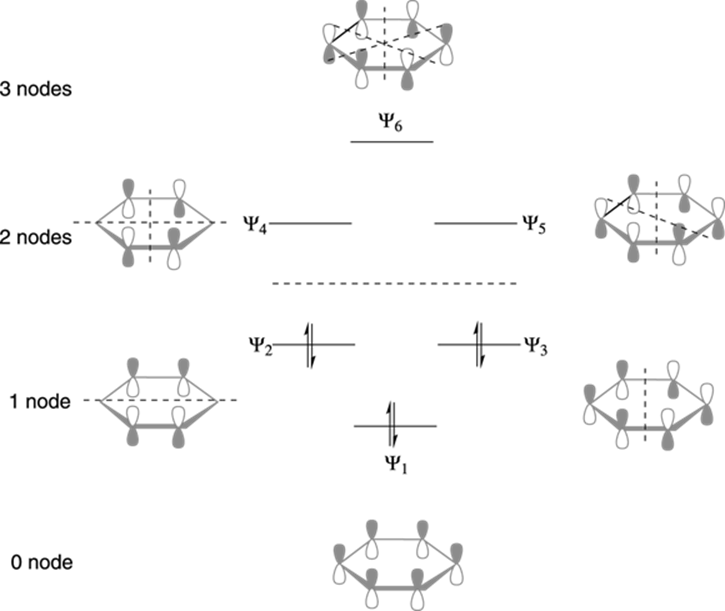
Orbitals with the same energy are described as degenerate orbitals. Quantum mechanical calculations tell us that the six pi molecular orbitals in benzene formed from six atomic p orbitals occupy four separate energy levels.
 Benzene Structure Chemistry Tutorvista Com
Benzene Structure Chemistry Tutorvista Com
The p orbital manifold is shown below.
Molecular orbital diagram of benzene. The structure of benzene molecule is best described in terms of molecular orbital treatment theory. Two electrons occupy the 1s orbital. In this molecule 24 of the 30 total valence bonding electrons24 coming from carbon atoms and 6 coming from hydrogen atomsare located in 12 σ sigma bonding orbitals.
In chemistry terms the highest occupied molecular orbitals homo of benzene are lower in energy than the highest occupied molecular orbital homo of hexatriene. Carbon has 6 electrons. Jeff quitney 588610 views.
The calculated energy of the molecular orbital is shown in the left corner. The atomic orbitals combine to produce the following molecular orbital diagram. The benzene ring is is regarded as a substituent when the parent chain has greater than six carbons.
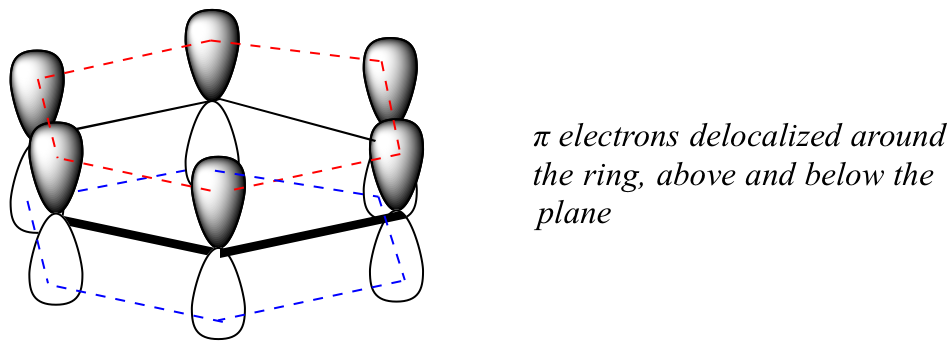
Three electrons participate in sp² bonds with the neighboring carbon atoms or with a hydrogen atom. Two sp2 hybrid orbitals of each c atom overlap with two sp2 hybrid orbital of two other c atoms to form sigma bonds. π molecular orbitals of benzene.
No one resonance forms accurately depicts the structure of the molecule. Introduction to molecular orbital theory. And for our purposes that lower energy of the pi electrons translates into lower reactivity.
According to this theory all the c atoms in benzene are sp2 hybridized. Note that the figure showing the molecular orbitals of benzene has two bonding π 2 and π 3 and two anti bonding π and π 5 orbital pairs at the same energy levels. An example is the mo description of benzene c 6h 6 which is an aromatic hexagonal ring of six carbon atoms and three double bonds.
Three bonding and three anti bonding. For bezene there is one coulomb term for each carbon atom with z6 and one coulomb term for each hydrogen atom with z1. When the benzene ring is a substituent of a parent chain referred to as a phenyl group.
Crystals 1958 alan holden bell laboratories pssc physical science study committee duration. The upper bonding degenerate pair of orbitals are the homos of benzene. Lets look at an energy diagram of the pi molecular orbitals in benzene.
Pi 1 and pi 6 have unique energy levels while the pi 2 pi 3 and pi 4 pi 5 pairs are degenerate meaning they are at the same energy level. Molecular orbitals of benzene.
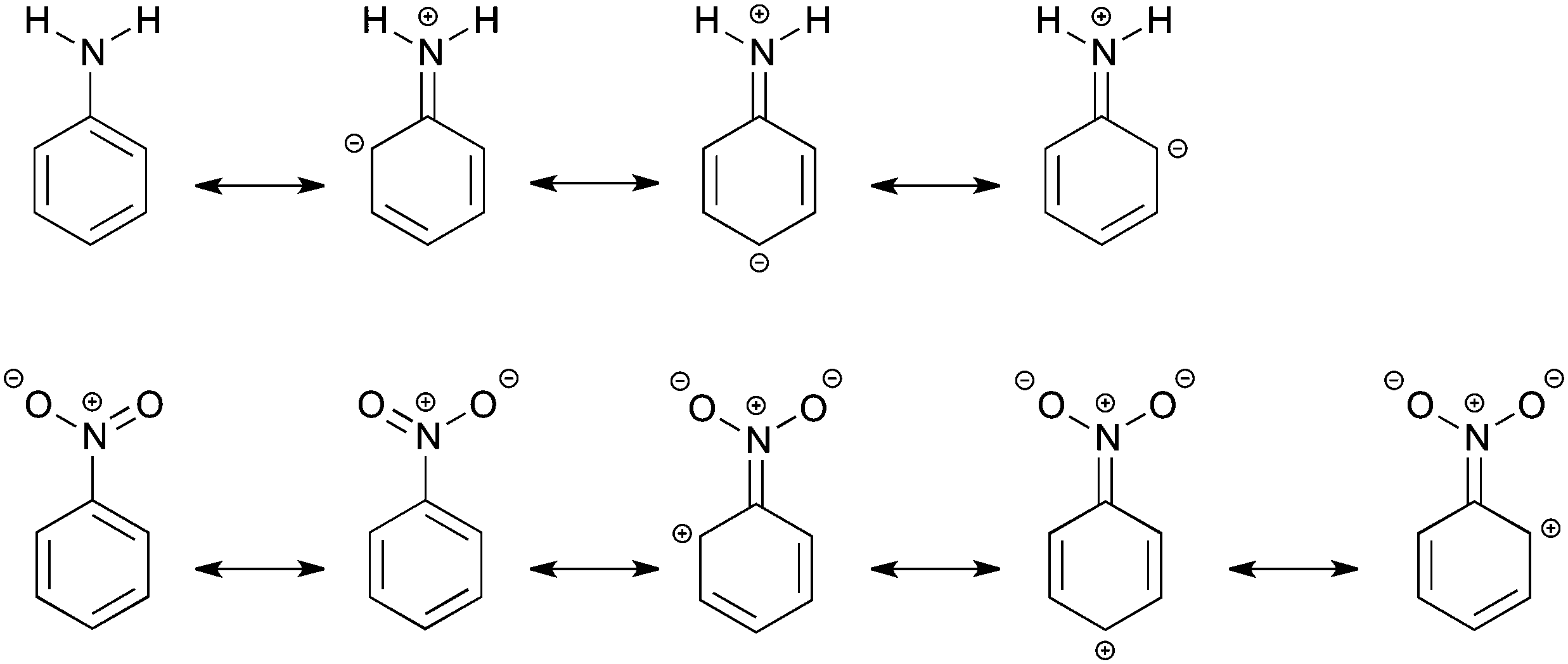
 How Amino And Nitro Substituents Direct Electrophilic Aromatic
How Amino And Nitro Substituents Direct Electrophilic Aromatic
Introduction To Molecular Orbital Theory
15 3 Pi Molecular Orbitals Of Benzene Chemistry Libretexts
Molecular Orbital Diagram Of Benzene Astonishing 2 2 Molecular
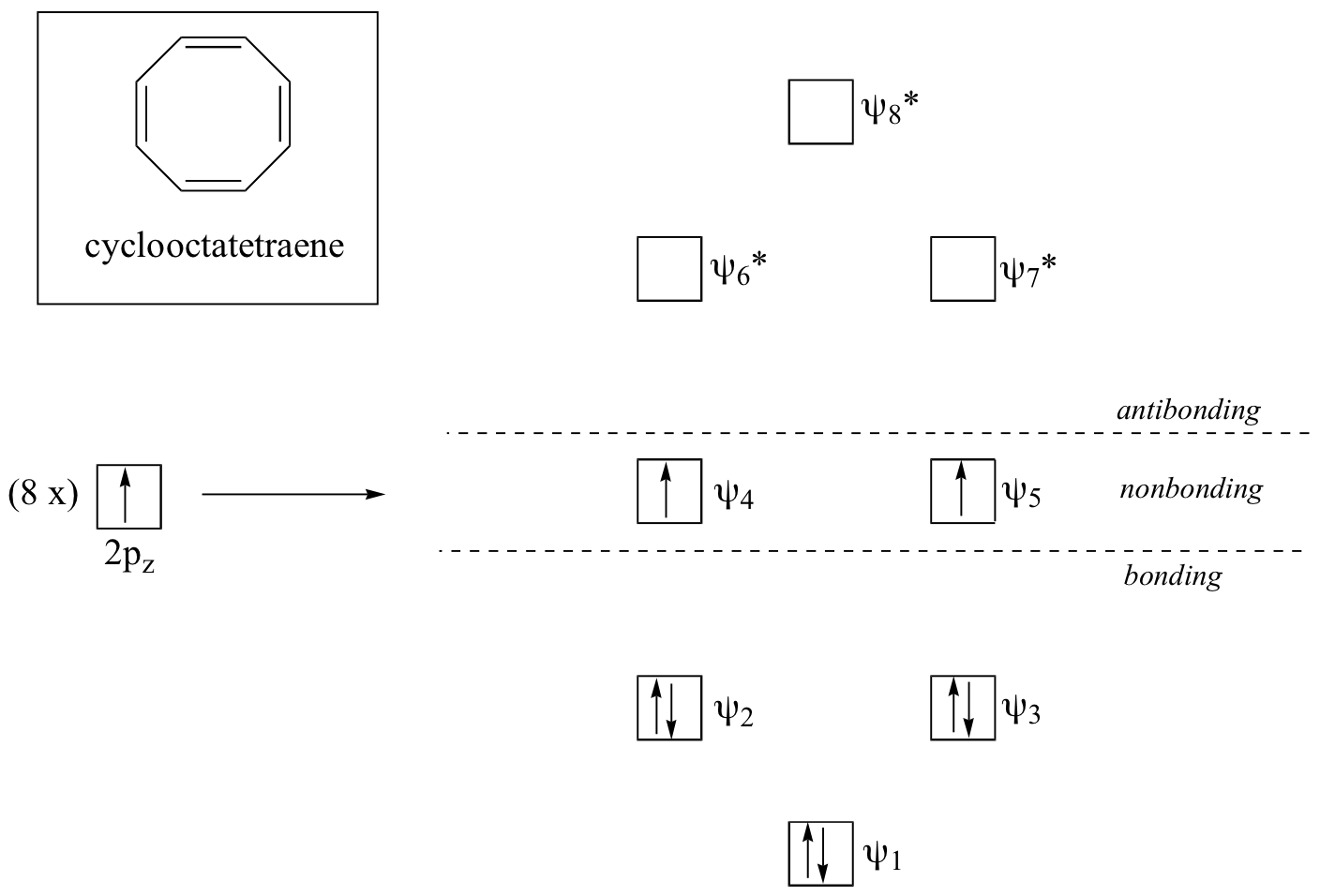 17 9 What Is The Basis Of Huckel S Rule Chemistry Libretexts
17 9 What Is The Basis Of Huckel S Rule Chemistry Libretexts
 Introduction To Molecular Orbital Theory Ppt Download
Introduction To Molecular Orbital Theory Ppt Download
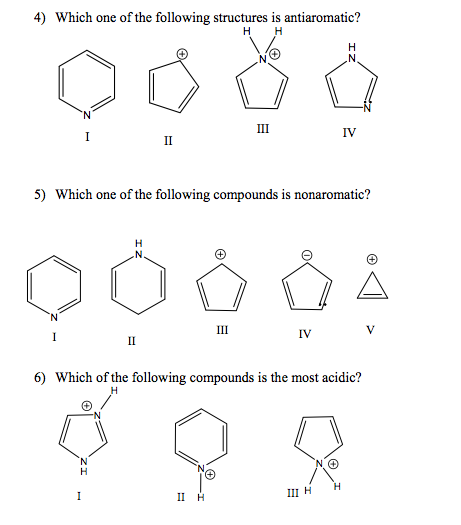 Solved According To Molecular Orbital Theory How Many
Solved According To Molecular Orbital Theory How Many
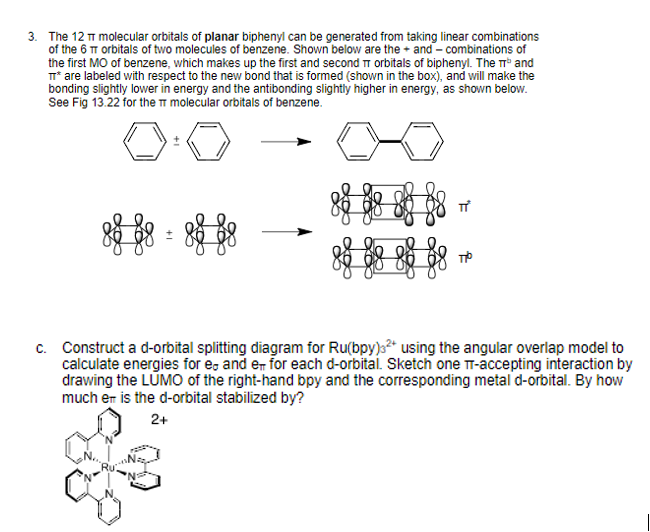 3 The 12 P Molecular Orbitals Of Planar Biphenyl Chegg Com
3 The 12 P Molecular Orbitals Of Planar Biphenyl Chegg Com
 Lord Of The Rings Aromatic Compounds Functional Groups Organic
Lord Of The Rings Aromatic Compounds Functional Groups Organic
 Molecular Orbitals Of Benzene 2 H Middle Compared To Those Of
Molecular Orbitals Of Benzene 2 H Middle Compared To Those Of
I Molecular Orbitals Of Conjugated Buta Ne Is Loaded Benzene Mo
 Orbital Picture Of Benzene Youtube
Orbital Picture Of Benzene Youtube
 14 Aromatic Compounds Approx Lecture Time 2 Lectures Topics
14 Aromatic Compounds Approx Lecture Time 2 Lectures Topics
 2 2 Molecular Orbital Theory Conjugation And Aromaticity
2 2 Molecular Orbital Theory Conjugation And Aromaticity
 The Pi Mos Of Benzene And Hexatriene Youtube
The Pi Mos Of Benzene And Hexatriene Youtube
 Figure 5 From Tri Tert Butylsily 1 Imido Complexes Of Titanium
Figure 5 From Tri Tert Butylsily 1 Imido Complexes Of Titanium
Molecular Orbital Description Of Stability Bonding Molecular
Introduction To Molecular Orbital Theory
 Benzene Structure Chemistry Tutorvista Com
Benzene Structure Chemistry Tutorvista Com
 Molecular Orbital Treatment Of Benzene Bond Lingth Analysis In
Molecular Orbital Treatment Of Benzene Bond Lingth Analysis In
 Schematic Molecular Orbital Diagram Of Homo And Lumo Energy Levels
Schematic Molecular Orbital Diagram Of Homo And Lumo Energy Levels
 Chapter 7 Electron Delocalization And Resonance More About Molecular
Chapter 7 Electron Delocalization And Resonance More About Molecular
0 Response to "Molecular Orbital Diagram Of Benzene"
Post a Comment