Which Diagram Shows Electrons Violating The Pauli Exclusion Principle
1s2 which diagram shows the correct distribution of electrons in the electron shells of a helium atom. Which one of the following represents an incorrect set of quantum numbers for an electron in an atom arranged as n l m l and m s.
 Pauli S Exclusion Principal Youtube
Pauli S Exclusion Principal Youtube
Pauli violation because the two electrons in the 3s have the same spin.

Which diagram shows electrons violating the pauli exclusion principle. Which diagram shows electrons violating the pauli exclusion principle. Full s orbital shields the electron in the p orbital from the nucleus. Based on these rules.
Thus it can be seen that in option c electrons in last 2 subshell have electrons with same spin which is a violation of pauli exclusion principle. When we draw electrons we use up and down arrows. Aufbau principle electrons fill orbitals starting at the lowest available energy state before filling higher states 1s before 2s.
An orbital can hold 0 1 or 2 electrons only and if there are two electrons in the orbital they must have opposite paired spins. Not mc014 4jpgmc014 5jpgmc014 6jpg up down up down up down. Paulis exclusion principle says that no two electrons can have the same quantum numbers so the maximum electrons that can occupy each orbital are 2 and they much have opposite spins.
To show the electron configuration for an atom what is the advantage of using an orbital notation compared to a dot structure. Orbital notation shows the spin of the electrons. How many electrons can the n 4 shell hold.
Which set of orbital diagrams shows a violation of the pauli exclusion principle. Fermions include elementary particles such as quarks the constituent particles of protons and neutrons electrons and neutrinos. Recognizing examples and non examples of the aufbau principle drawing electron configurations with aufbauorbital diagram finding violations of pauli exclusion principle hunds rule and aufbau principle in diagrams identifying elements that are paramagnetic or diamagnetic recognizing excited versus ground states.
The pauli exclusion principle governs the behavior of all fermions particles with half integer spin while bosons particles with integer spin are not subject to it. Atomic radii of group 13 elements are generally bigger. Further an orbital can contain a maximum of only two electrons the two electrons must have opposing spins.
What is the maximum number of electrons that can occupy the subshell 3d. The electron configuration for helium he is shown below. Chemistry chapter 2 quiz 6.
One should be pointing down. Electrons in group 13 elements are closer to the nucleus.
 C Curceanu Petrascu S Research Works Politecnico Di Milano
C Curceanu Petrascu S Research Works Politecnico Di Milano
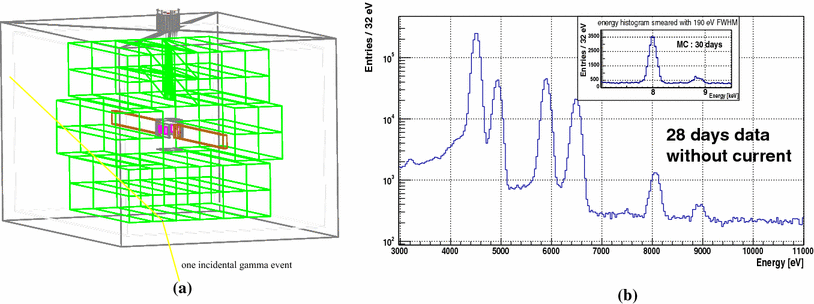 Experimental Search For The Violation Of Pauli Exclusion Principle
Experimental Search For The Violation Of Pauli Exclusion Principle
 Pdf Vip An Experiment To Search For A Violation Of The Pauli
Pdf Vip An Experiment To Search For A Violation Of The Pauli
Exchange Antisymmetry And Pauli Repulsion
 Violation In Electrons E Of The 3 8 Pc For The Li Atom And The H3
Violation In Electrons E Of The 3 8 Pc For The Li Atom And The H3
 Which Electron Configuration Represents A Violation O
Which Electron Configuration Represents A Violation O
 A Violation Of The Pauli Exclusion Principle May Be The Easiest To
A Violation Of The Pauli Exclusion Principle May Be The Easiest To
Is Pauli S Exclusion Principle Applicable To A Photon Quora
Comments On Testing Charge Conservation And The Pauli Exclusion
New Limits On Bosonic Dark Matter Solar Axions Pauli Exclusion
 Solved Shown Below Are Portions Of Orbital Diagrams Repre Sen
Solved Shown Below Are Portions Of Orbital Diagrams Repre Sen
 Dublin Schools Lesson Orbital Diagrams And Electron Configurations
Dublin Schools Lesson Orbital Diagrams And Electron Configurations
 Testing Noncommutative Spacetimes And Violations Of The Pauli
Testing Noncommutative Spacetimes And Violations Of The Pauli
Lectures 10 11 Multi Electron Atoms Schrodinger Equation For
Pauli Exclusion Principle Violates Energy Conservation

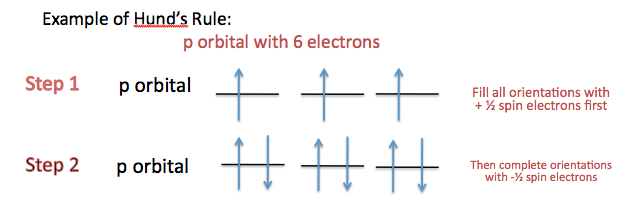 12 10 Electron Spin And The Pauli Principle Chemistry Libretexts
12 10 Electron Spin And The Pauli Principle Chemistry Libretexts
 Atomic Structures Pauli Exclusion Principle Aufbau Principle
Atomic Structures Pauli Exclusion Principle Aufbau Principle
 Pauli Exclusion Principle Violates Energy Conservation
Pauli Exclusion Principle Violates Energy Conservation
 Pauli Exclusion Principle Chemistry Libretexts
Pauli Exclusion Principle Chemistry Libretexts
 Atomic Structures Pauli Exclusion Principle Aufbau Principle
Atomic Structures Pauli Exclusion Principle Aufbau Principle
0 Response to "Which Diagram Shows Electrons Violating The Pauli Exclusion Principle"
Post a Comment