Examine The Following Phase Diagram And Determine What Phases Exists At Point A
Ios android web. B london dispersion forces.
The normal boiling point of ether is 3078 k.
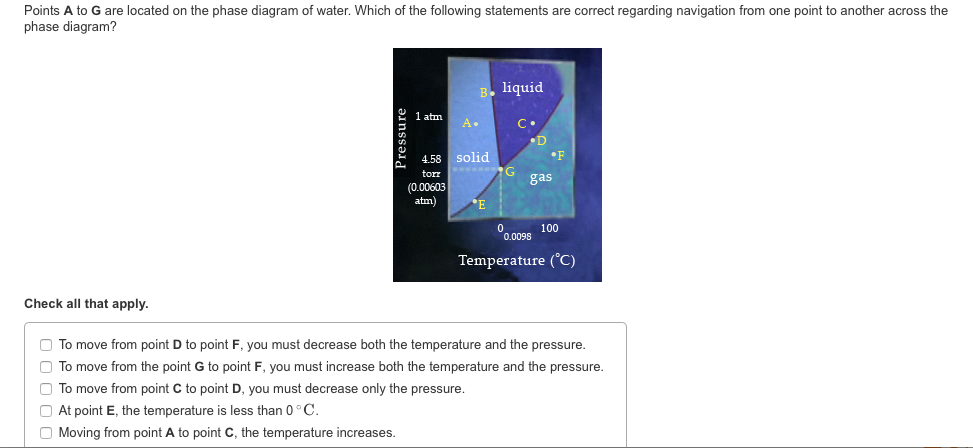
Examine the following phase diagram and determine what phases exists at point a. Chem 1412 su06 exam 1. A dispersion forces b dipole dipole interactions c dipole. Examine the following phase diagram and identify the feature represented by point a.
Examine the following phase diagram and determine what phase exists at point f. Examine the following phase diagram and determine what phase exists at point fa vapor liquidb vaporc liquidd solide supercritical fluid. Examine the following phase diagram and determine what phase exists at point f.
The heat of vaporization for either is 2669 kjmol. Examine the following phase diagram and identify the feature represented by point b. Examine the phase diagram for the substance bogusium bo and select the correct statement.
C bo changes from a solid to a liquid as one follows the line from c to d. This preview has intentionally blurred sections. Formed by joining n type and p type semiconductor materials as shown below.
Examine the following phase diagram and determine what phase exists at point favapor liquidb. Since the n type region has a high electron concentration and the p type a high hole concentration electrons diffuse from the n type side to the p type side. Vapor which of the following intermolecular forces is the weakest.
Increasing temperature with a phase change from solid to vapor examine the following phase diagram and determine what phase exists at point f. Circuit elements that allow the flow of electrical current in only one direction. A vapor liquid b vapor c liquid d solid e supercritical fluid 4.
B the triple point for bo is at a higher temperature than the melting point for bo. White fall 2013 10. Sign up to view the full version.
Examine the following phase diagram and determine. Eincreasing temperature beyond the critical point 7. Solved by professors experts.
A bos has a lower density than bol. Examine the following phase diagram and identify the feature represented by point a and point b. Examine the following phase diagram and identify the feature represented by point a.
Avapor liquid bvapor cliquid dsolid esupercritical fluid 3. Neon condenses due to a dipole dipole forces. Chapter 12 consider the following phase diagram and identify the process occurring as one goes from point c to point d.
Calculuate the temperature at which its vapor pressure is exactly half of that at its normal boiling point. Examine the phase diagram for the substance bogusium bo and select the correct statement.
 A Possible Four Phase Coexistence In A Single Component System
A Possible Four Phase Coexistence In A Single Component System
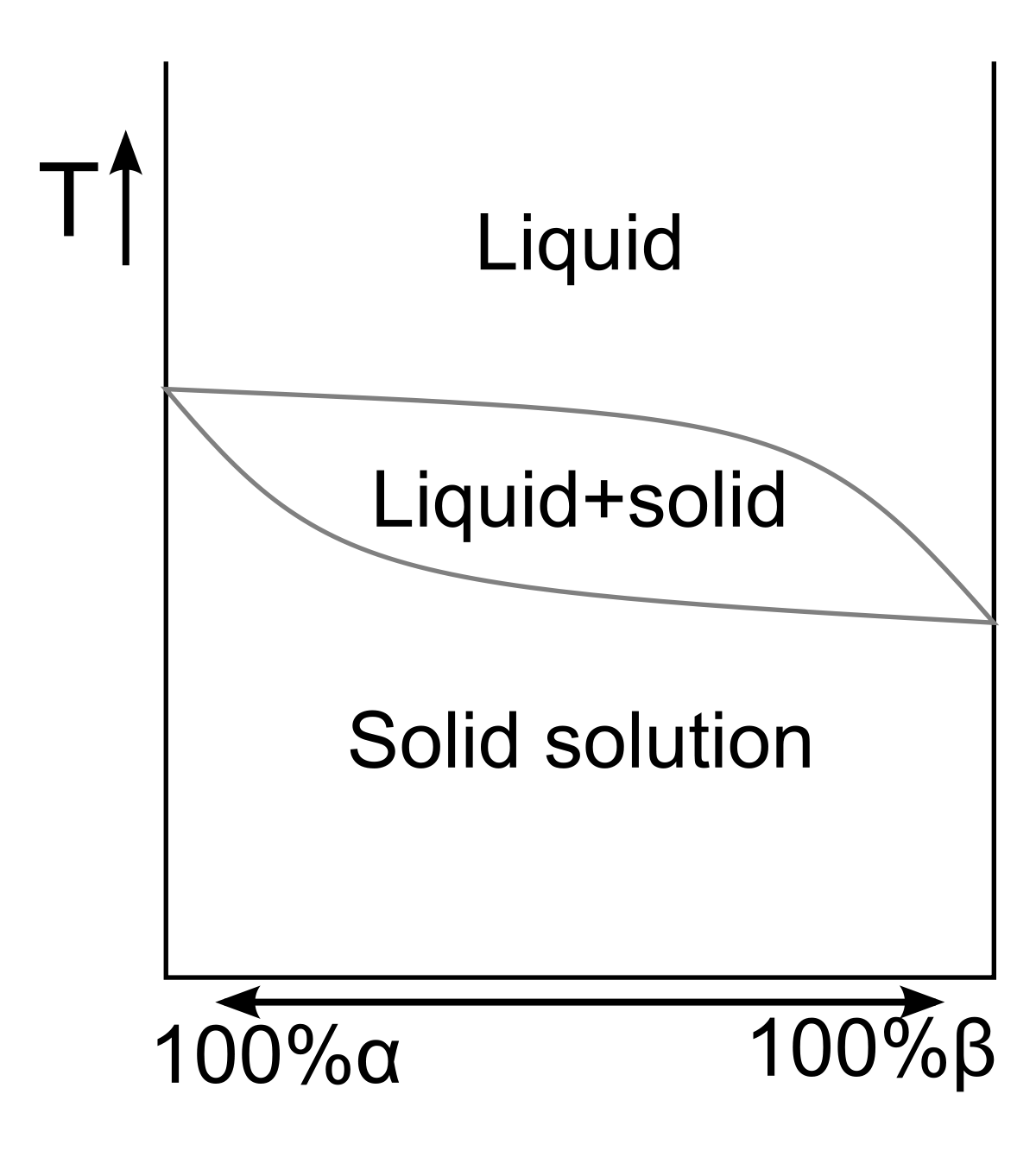
Lever Rule Applied To Phase Diagram For Partially Miscible Liquids
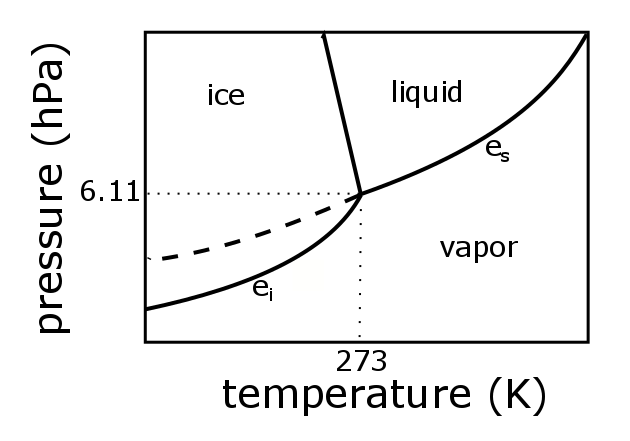 3 3 Phase Diagram For Water Vapor Clausius Clapeyron Equation
3 3 Phase Diagram For Water Vapor Clausius Clapeyron Equation
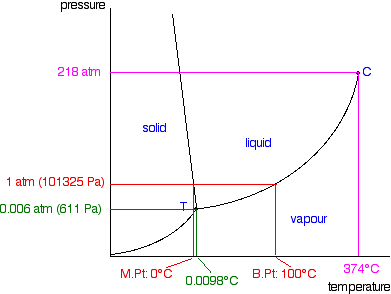 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
Phase Transformations And Phase Diagrams Substech
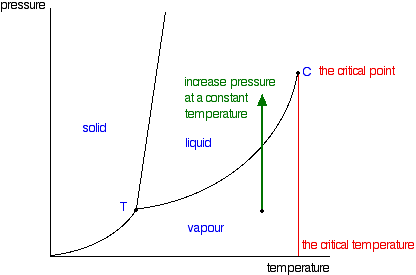 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
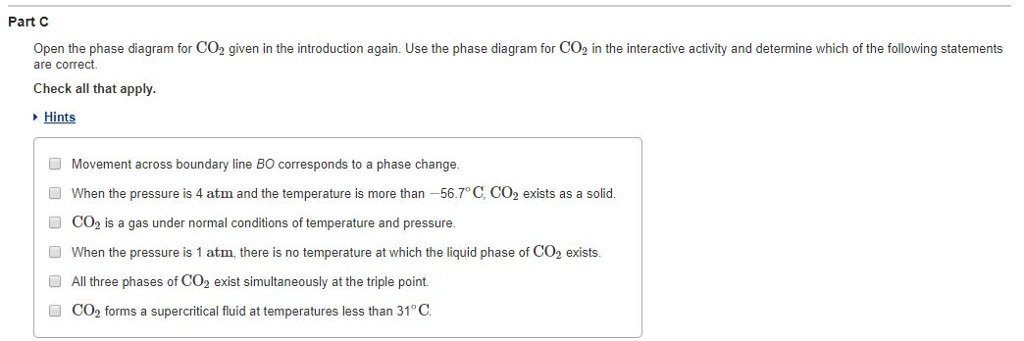 Solved Part C Open The Phase Diagram For Co2 Given In The
Solved Part C Open The Phase Diagram For Co2 Given In The
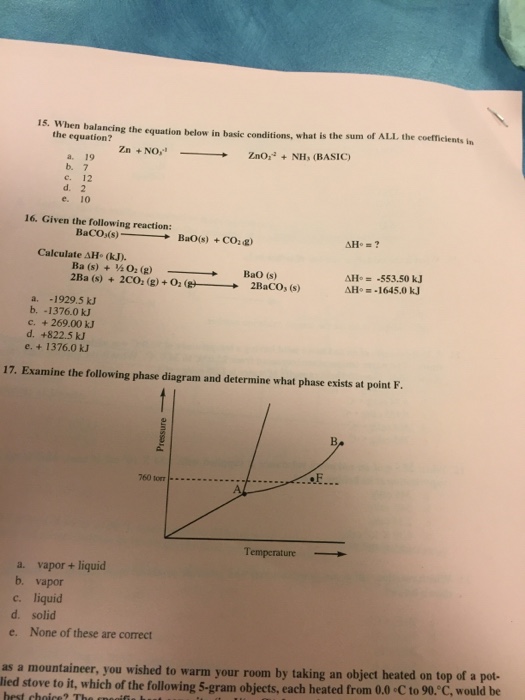
 Solved A Phase Diagram Is A Temperature Pressure Plot Tha
Solved A Phase Diagram Is A Temperature Pressure Plot Tha


0 Response to "Examine The Following Phase Diagram And Determine What Phases Exists At Point A"
Post a Comment