What Is The Basis For Exceptions To The Aufbau Diagram
But because half filled orbitals and complete orbitals are more stable an electron from the s sublevel will transfer to the d sublevel. Aufbau diagrams for a lithium ion.
 Clarifying Electron Configurations Chemical Education Xchange
Clarifying Electron Configurations Chemical Education Xchange
Chemistry 1 chapter 5 test review.

What is the basis for exceptions to the aufbau diagram. Some elements have unusual atomic orbitals share to. In the lower atomic numbers the difference in energy levels for the normal sequence of electron shells is larger and exceptions are not as common. For example for cu with z29.
Because the promotion energy is lower than the spin pairing energy it is much easier for the electron to become promoted to the p orbital rather than spin and pair in the s orbital. A chart or diagram may be used to show how the principle works for various example elements. Region of high probability of finding an electron.
Titanium the fourth period of the periodic table contain. 1s 2s 2p 3s 3p. What is the basis for exceptions to the aufbau diagram.
A filled and half filled energy sublevels are more stable than partially filled energy sublevels b electron configurations are only probable. What is the basis for exceptions to the aufbau diagram. Other exceptions are copper and silver.
What is the basis for exceptions to the aufbau diagram. In the first 30 elements only copper atomic number 24 and chrome atomic number 29 are exceptions to the aufbau principle. Filled and half filled energy sublevels are more stable than partially filled energy sublevels.
Copper and chromium are exceptions to the aufbau rule because they both have a higher spin energy than promotion energy. Copper and chromium are exceptions to the aufbau rule because they both have a higher spin energy than promotion energy. Learn vocabulary terms and more with flashcards games and other study tools.
Because the promotion energy is lower than the spin pairing energy it is much easier for the electron to become promoted to the p orbital rather than spin and pair in the s orbital. Despite the exceptions the aufbau principle is useful in chemistry courses where students discover the fundamental rules about the atomic structure and properties of elements. K ca sc ti v cr mn fe co ni cu zn ga ge as se br kr.
What is the basis for exceptions to the aufbau diagrams. In bohrs model of the atom where are the electrons and protons located. What is the basis for exceptions to the aufbau diagram.
Chapter 5 review electrons in atoms chapter 5 review what is the next atomic orbital in the series. What is the fourth element in the fourth period of the periodic table of elements. How does the energy of an electron change when the electron moves closer to the nucleus.
Thus ar 4s¹ 3d¹⁰. Start studying chemistry chapter 5. Here the s sublevel is half filled and the d sublevel is completely filled.
If we follow aufbau its condensed electronic config should be ar 4s² 3d⁹.
 8 3 Electron Configurations How Electrons Occupy Orbitals
8 3 Electron Configurations How Electrons Occupy Orbitals
A Linear Cobalt Ii Complex With Maximal Orbital Angular Momentum
 Pauli Exclusion Principle An Overview Sciencedirect Topics
Pauli Exclusion Principle An Overview Sciencedirect Topics
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
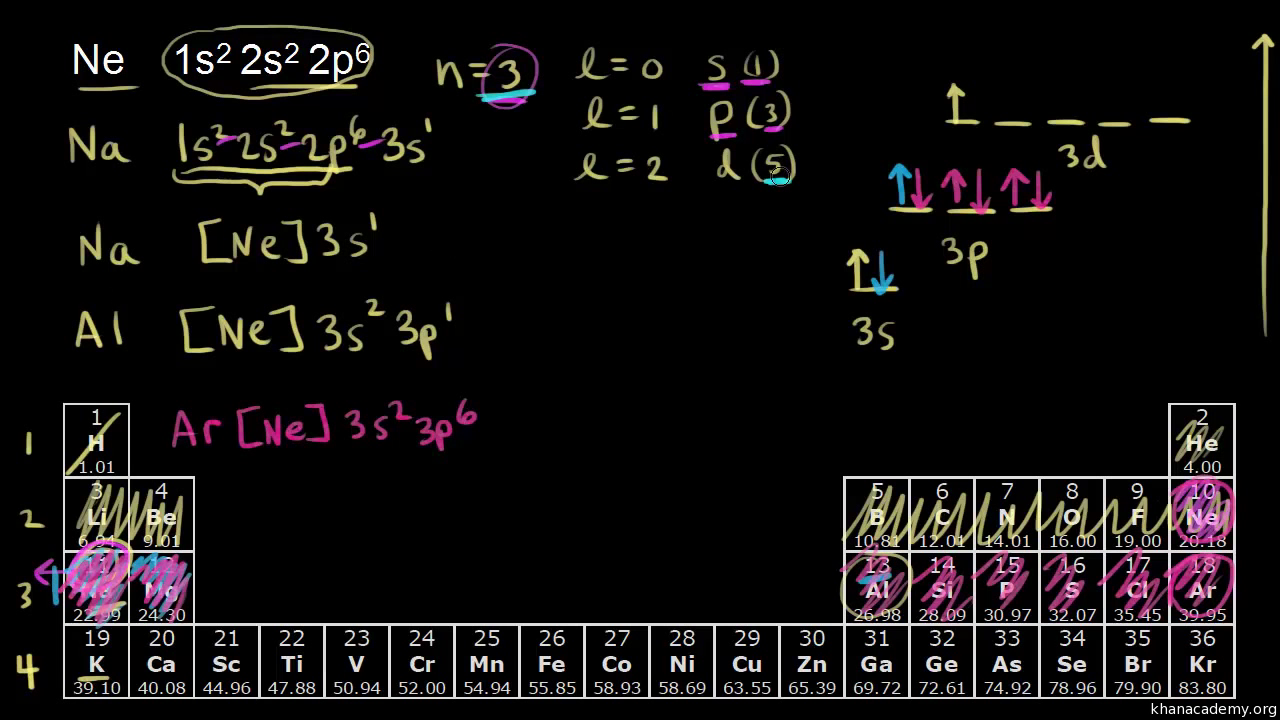 Electronic Structure Of Atoms Chemistry Science Khan Academy
Electronic Structure Of Atoms Chemistry Science Khan Academy
 Electron Configuration Wikipedia
Electron Configuration Wikipedia
A Linear Cobalt Ii Complex With Maximal Orbital Angular Momentum
 Many Electron Systems Book Chapter Iopscience
Many Electron Systems Book Chapter Iopscience
 Basis Set Convergence Errors In The Dissociation Energy D E Bs
Basis Set Convergence Errors In The Dissociation Energy D E Bs
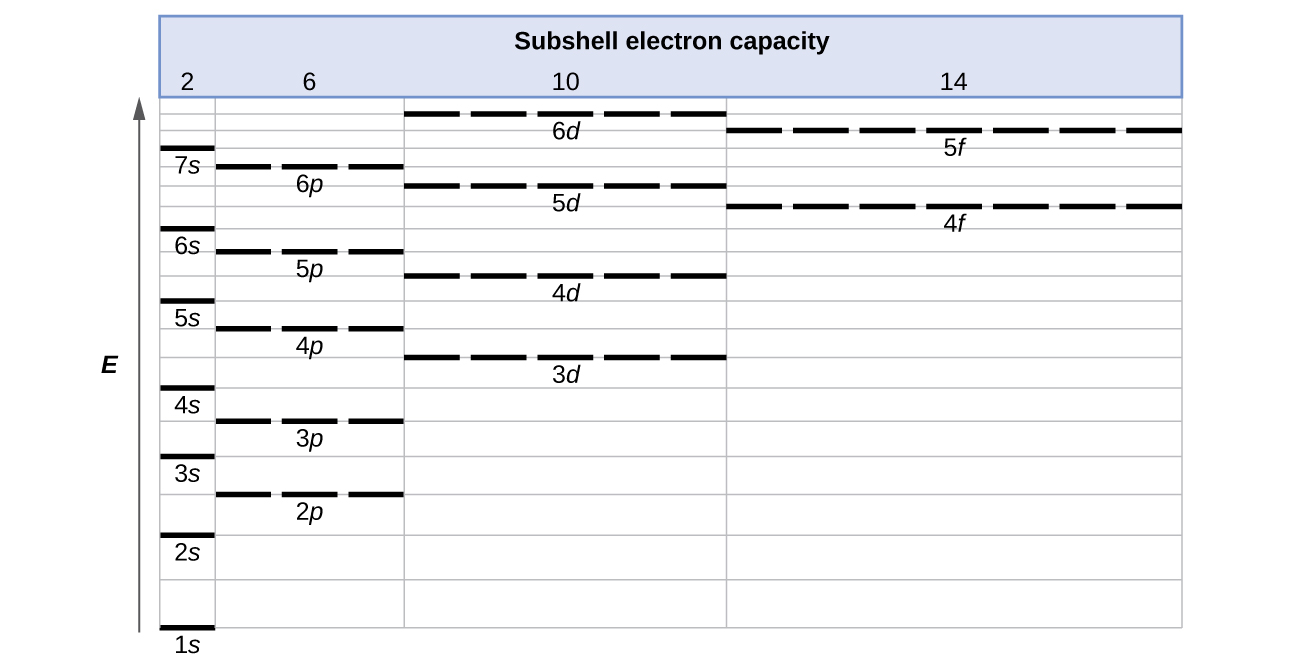 3 1 Electron Configurations Chemistry Libretexts
3 1 Electron Configurations Chemistry Libretexts
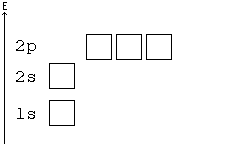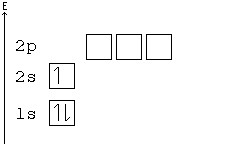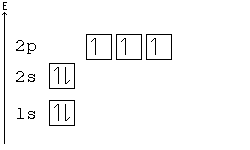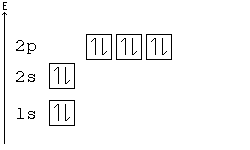 General Chemistry Filling Electron Shells Wikibooks Open Books
General Chemistry Filling Electron Shells Wikibooks Open Books
 Energy Consistent Relativistic Pseudopotentials And Correlation
Energy Consistent Relativistic Pseudopotentials And Correlation
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
 How Do Chromium And Copper Contradict The Aufbau Principle Quora
How Do Chromium And Copper Contradict The Aufbau Principle Quora
 The Trouble With The Aufbau Principle Feature Education In Chemistry
The Trouble With The Aufbau Principle Feature Education In Chemistry
 What Is The Basis For Exceptions To The Aufbau Principle Sciencing
What Is The Basis For Exceptions To The Aufbau Principle Sciencing
 Multi Electron Atoms And The Periodic Table Book Chapter Iopscience
Multi Electron Atoms And The Periodic Table Book Chapter Iopscience
 Pdf The Hyperbola Of Quantum Chemistry The Changing Practice And
Pdf The Hyperbola Of Quantum Chemistry The Changing Practice And
 Electron Configuration Wikipedia
Electron Configuration Wikipedia
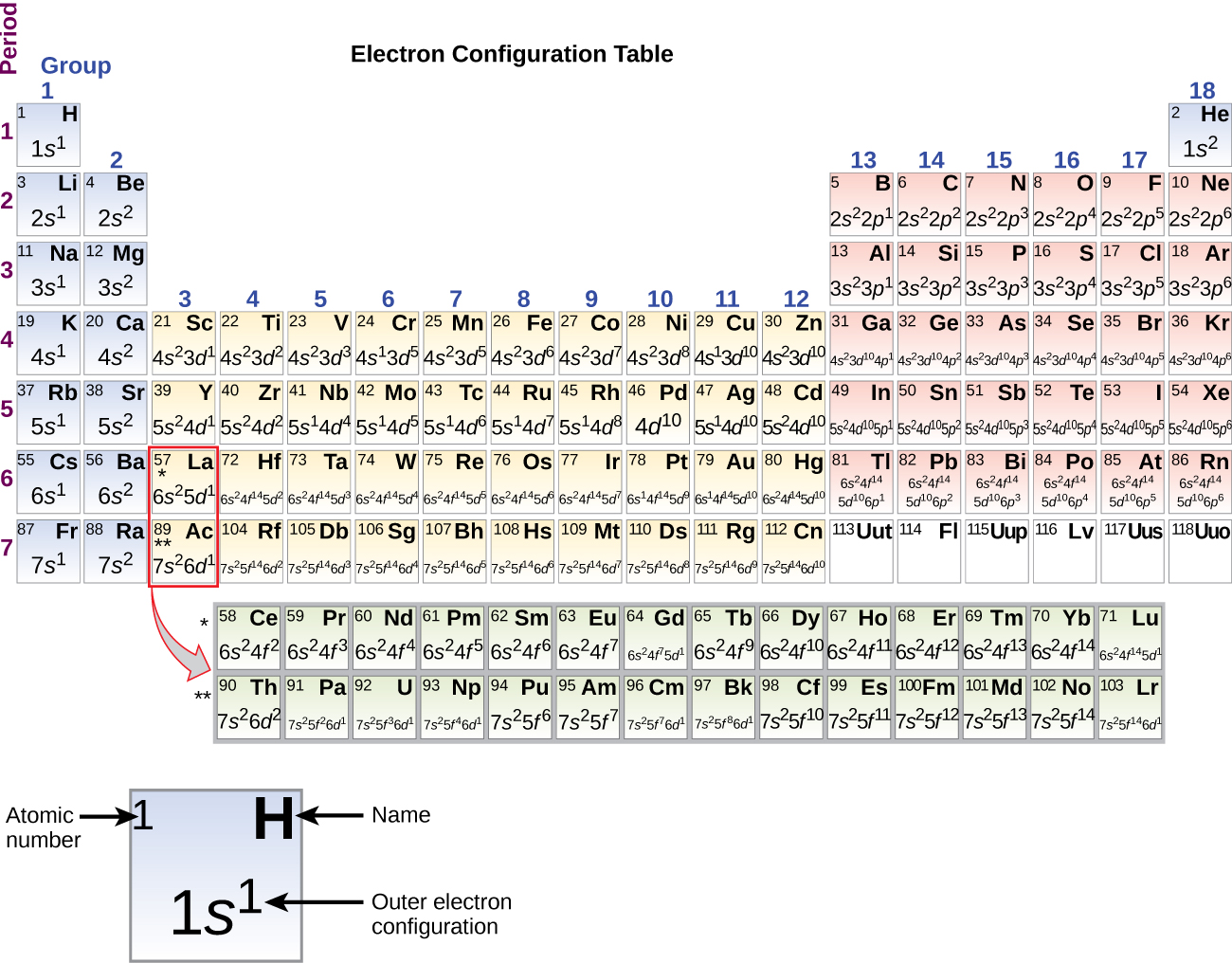 8 3 Electron Configurations How Electrons Occupy Orbitals
8 3 Electron Configurations How Electrons Occupy Orbitals
0 Response to "What Is The Basis For Exceptions To The Aufbau Diagram"
Post a Comment