Molecular Orbital Diagram Of H2
Because of their simplicity they have been extensively studied. Bonding order is 1 and it is diamagnetic.
Mo diagrams can explain why some molecules exist and others do not.
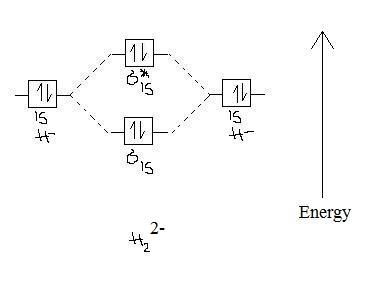
Molecular orbital diagram of h2. The lewis structure for h2 is h h predicting a single bond between each hydrogen atom with two electrons in the bond. Ab a b gg with. The orbital correlation diagram in predicts the same thing two electrons fill a single bonding molecular orbital.
Molecular orbital mo theory of the h2 molecule. Molecular orbitals of h 2 the hydrogen atom is the simplest atom and its molecule ceh2 is also the simplest molecule than monoatomic molecules such as cehe and cene etc. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h2.
Molecular orbital diagram for hydrogen gas h2. Discussed in this video are. Because the energy of the two electrons is lower than the energy of the individual atoms the molecule is stable.
Molecular orbital diagrams of diatomic molecules. Here is the result obtained for egs r via eq. When two hydrogen atoms come closer then on combining two 1s orbitalstwo molecular orbitals are formed among which one is bonding and other is antibonding molecular.
Fill from the bottom up with 2 electrons total. A the molecular orbital energy level diagram for the h2 molecule. In order to predict the bond order molecular orbital diagram for h2 is to be drawn.
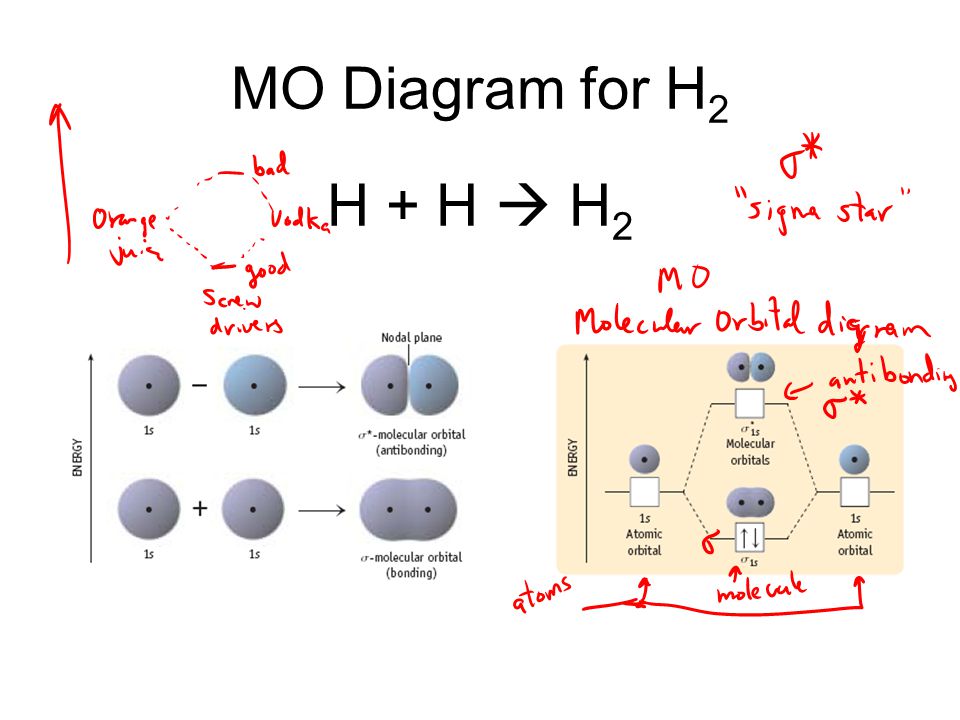
This tool is very well suited for simple diatomic molecules such as dihydrogen dioxygen and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules such as methane. Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. In phase and out of phase wave combinations.
Bonding mos antibonding mos and bond order. Sketch the molecular orbitals of the h2 ion and draw its energy level diagram. Suppose that the ion is excited by light so that an electron moves from a lower energy to a higher energy molecular orbital.
B the shapes of the molecular orbitals are obtained by squaring the wave functions for mo1 and mo2. Atomic valence electrons shown in boxes on the left and right fill the lower energy molecular orbitals before the higher ones. In fact they do.
According to mot number of atomic orbitals combined is equal to total number of molecular orbitals formedelectronic configuration of h is 1s1. Evaluate the ground state electronic energy based on this presumed approximate eigenfunction. πε and jr k r mr lr defined explicitly in atkins.
 Molecular Orbital Theory Ppt Download
Molecular Orbital Theory Ppt Download
 Orbital Bond Molecular Then Identify Construct Diagram H2 Order And
Orbital Bond Molecular Then Identify Construct Diagram H2 Order And
 Molecular Orbital Mo Diagram Of H2 Youtube
Molecular Orbital Mo Diagram Of H2 Youtube
 2 3b Mo Theory Of Bonding In H Chemistry Libretexts
2 3b Mo Theory Of Bonding In H Chemistry Libretexts
What Is The Molecular Orbital Diagram Of Hydrogen Quora
Ch4 Mo Diagram 9 7 Stromoeko De
Introduction To Molecular Orbital Theory
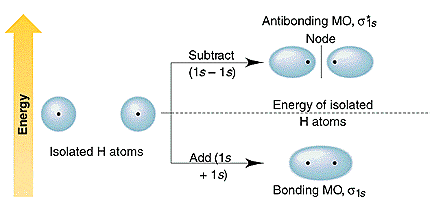 Molecular Orbitals Of H2 Chemistry Libretexts
Molecular Orbitals Of H2 Chemistry Libretexts
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
50 Awesome H2 Molecular Orbital Diagram Abdpvtltd Com
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 Bonding In Homonuclear Diatomic Molecules H2 H2 H2 He2 He2
Bonding In Homonuclear Diatomic Molecules H2 H2 H2 He2 He2
Construct The Molecular Orbital Diagram For H2 And Then Identify
Introduction To Molecular Orbital Theory
Molecular Orbital Diagram For He2
Is H2 A Viable Molecule For The Molecular Orbital Theory Quora
Introduction To Molecular Orbital Theory
Sparknotes Organic Chemistry Orbitals Problems Molecular Orbital

0 Response to "Molecular Orbital Diagram Of H2"
Post a Comment