How To Draw A Molecular Orbital Diagram
The number of molecular orbitals in the diagram must equal the number of atomic orbitals combined eg in li 2 two atomic orbitals 1s and 2s from each atom are combined to make four molecular. Label homo and lumo and using dashed lines draw the missing nodes.
 Organic Chemistry Draw A Simplified Mo Diagram For The Pi System
Organic Chemistry Draw A Simplified Mo Diagram For The Pi System
Provide a molecule whose molecular orbital diagram matches the one provided.
How to draw a molecular orbital diagram. Draw the molecular orbitals not energy level diagram of a ethane and b acetylene and show how the cc and ch σ bonds and cc π bonds are. Fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. Energy is on the y axis.
Examples and tricks for drawing lewis dot diagrams of molecules duration. You can see that the antibonding orbitals are higher in energy and bonding orbitals are lower in energy. Two atomic orbitals in phase create a larger electron density which leads to the σ orbital.
So 2 electrons on σ2s. Based on the amount of orbital overlap the relative changes in energy differ going from the atomic orbital to the molecular orbital. Molecular orbital diagram of oxygen molecule nature of chemical bond.
The following factors contribute to the position of one mo with respect to other mos. To draw a molecular orbital mo diagram you need to consider which atomic orbitals aos the molecule has. Greater overlap greater change in energy.
If the two 1s orbitals are not in phase a node between them causes a jump in energy the σ orbital. This is the general mo diagram you need to fill with the valence electrons of bn. Inside the dashed lines are the possible molecular orbitals they are capable of forming.
Drawing molecular orbitals additional practice problems. Molecular orbital theory posits the notion that electrons in molecules likewise exist in different orbitals that give the probability of finding the electron at particular points around the molecule. The regions to the left and right side of the dashed lines are atomic orbitals.
Atomic orbital diagram for oxygen atom oxygen atom is on period 2 so it has access to its 1s 2s and 2p aos. Thus we take 10 atomic orbitals and generate 10 molecular orbitals in accordance with the conservation of orbitals. Boron has 3 valence electrons and nitrogen has 5 valence electrons this makes 8 electrons.
Keep in mind the energy of the atomic orbitals and molecular orbitals. To produce the set of orbitals for a molecule we add together the valence atomic wavefunctions for the bonded atoms in the molecule. You have to start filling the orbitals from those with lowest energy to those with higher energy.
The superposition of the two 1s atomic orbitals leads to the formation of the σ and σ molecular orbitals.
 Solved Question 1 By Drawing Molecular Orbital Diagrams
Solved Question 1 By Drawing Molecular Orbital Diagrams
 Mo Diagram B2 7 2 Stromoeko De
Mo Diagram B2 7 2 Stromoeko De
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
 Solved A Draw A Molecular Orbital Diagram For The Nitric
Solved A Draw A Molecular Orbital Diagram For The Nitric
 How To Draw Overlapping Of Pure Or Hybridized Orbitals For Br2 And
How To Draw Overlapping Of Pure Or Hybridized Orbitals For Br2 And
Which Is The Molecular Orbital Diagram For Hf Quora
How To Draw Molecular Orbital Diagram Molecular Orbital Diagram Cn
Sparknotes Organic Chemistry Orbitals Problems Molecular Orbital
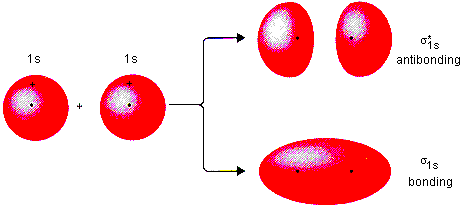 Molecular Orbital Diagrams Simplified Megan Lim Medium
Molecular Orbital Diagrams Simplified Megan Lim Medium
 Mo Diagram B2 7 2 Stromoeko De
Mo Diagram B2 7 2 Stromoeko De
Molecular Orbital Diagram Wikipedia
 How To Draw Molecular Orbital Diagram 67598 10 8 Molecular Orbital
How To Draw Molecular Orbital Diagram 67598 10 8 Molecular Orbital
 Advanced Inorganic Chemistry Nh3 Molecular Orbitals Wikibooks
Advanced Inorganic Chemistry Nh3 Molecular Orbitals Wikibooks
 Molecular Orbital Diagram For A Simple Pi Bond Bonding And
Molecular Orbital Diagram For A Simple Pi Bond Bonding And
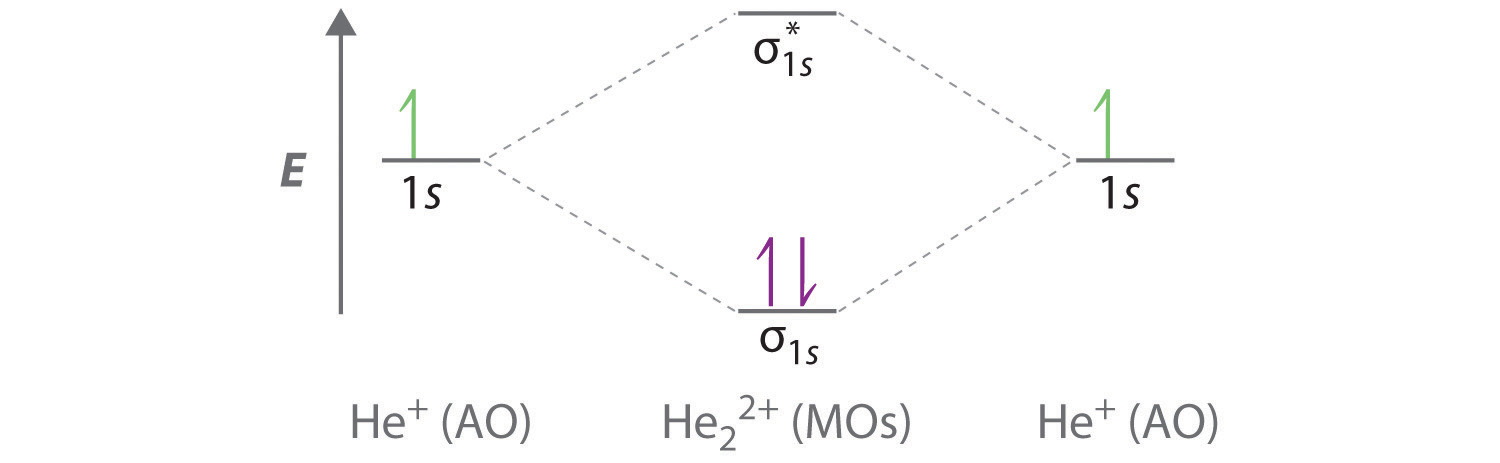 11 5 Molecular Orbital Theory Chemistry Libretexts
11 5 Molecular Orbital Theory Chemistry Libretexts
Molecular Structure Atomic Orbitals
 Molecular Orbital Diagram For A Simple Pi Bond Bonding And
Molecular Orbital Diagram For A Simple Pi Bond Bonding And
 12 E The Chemical Bond Exercises Chemistry Libretexts
12 E The Chemical Bond Exercises Chemistry Libretexts
 Constructing The O2 Molecular Orbital Energy Level Diagram Youtube
Constructing The O2 Molecular Orbital Energy Level Diagram Youtube
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
Introduction To Molecular Orbital Theory
0 Response to "How To Draw A Molecular Orbital Diagram"
Post a Comment