Lewis Dot Diagram For N
Electron configuration into shells. More complicated versions can be used to show the bond between different atoms in a molecule.
The lewis structure of a positive ion cation is positioned adjacent to the lewis structure of a negative ion anion.

Lewis dot diagram for n. How to draw lewis dot structures. However these structures are helpful in understanding the. A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Lewis who introduced it in his 1916 article the atom and the molecule. After that i draw the lewis dot structure for nitrogen n. These diagrams are used as a shorthand notation to show the number of valence electrons in an atom.
The first shell n1 can have only 2 electrons so that shell is filled in helium the first noble gas. The number of dots equals the number of valence electrons in the atom. Electron dot diagrams sometimes called lewis dot diagrams were first used by gilbert n.
Lewis dot diagrams of selected elements. Lewis electron dot diagrams for ions have less for cations or more for anions dots than the corresponding atom. Exercises explain why the first two dots in a lewis electron dot diagram are drawn on the same side of the atomic symbol.
Drawing lewis dot structures also known as lewis structures or lewis diagrams can be confusing particularly for a beginning chemistry student. Since it is in group 5 it will have 5 valence electrons. Index chemical concepts chemistry of the elements.
In the periodic table the elements are placed in periods and arranged left to right in the. Nitrogen is in group 5 sometimes called group v or group 15. Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.
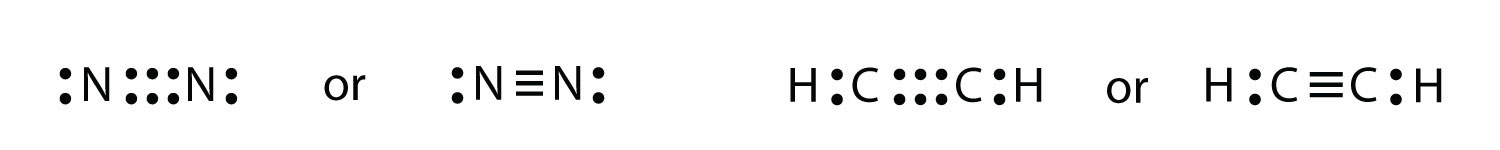
Put brackets and a negative sign around the n 3 lewis structure to show that it is an ion. The lewis structure was named after gilbert n. With n 3 youll need to form two double bonds between the nitrogen atoms to fill the octets and still use only the 34 valence electrons available for the molecule.
The lewis structure electron dot diagram of each ion is used to construct the lewis structure electron dot diagram for the ionic compound.
 Covalent Bonding And Lewis Structures
Covalent Bonding And Lewis Structures
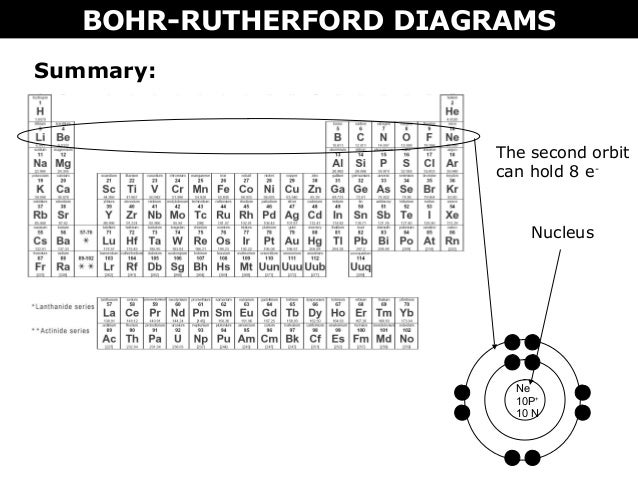 02 A Bohr Rutherford Diagrams And Lewis Dot Diagrams
02 A Bohr Rutherford Diagrams And Lewis Dot Diagrams
 Lewis Structure Dot Structure Of No2 Chemistry Net
Lewis Structure Dot Structure Of No2 Chemistry Net
 N Dot Diagram 5 18 Stromoeko De
N Dot Diagram 5 18 Stromoeko De
Lewis Structure Of N2f4 Biochemhelp
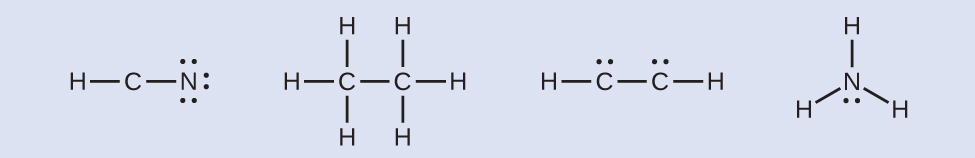 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
Orbital Diagram For Nitrogen Iodine Electron Dot Diagram
Solved What Is The Electron Dot Diagram For Nitrogen Fixya
 What Is The Lewis Structure Of N2 Socratic
What Is The Lewis Structure Of N2 Socratic
 Diagram For Nitrogen Valence Chapter 1 Structures And Bonding
Diagram For Nitrogen Valence Chapter 1 Structures And Bonding
 Dot Diagram For Al3 Wiring Schematic Diagram
Dot Diagram For Al3 Wiring Schematic Diagram
What Is The Formation Of A Ch4 Molecule With The Lewis Dot Structure
 High School Chemistry Lewis Electron Dot Diagrams Wikibooks Open
High School Chemistry Lewis Electron Dot Diagrams Wikibooks Open
 What Is The Lewis Dot Structure For N2 Quora
What Is The Lewis Dot Structure For N2 Quora
The Chemistry Of Nitrogen And Phosphorous
 Solved Draw The Lewis Dot Structure Of N2h4 What Is The
Solved Draw The Lewis Dot Structure Of N2h4 What Is The
Aluminum Nitrogen 3 Aln Chemspider
 Hcn Lewis Structure Molecular Geometry Shape And Polarity
Hcn Lewis Structure Molecular Geometry Shape And Polarity
Lewis Dot Diagram For Calcium Atom Best Wiring Library
 Electron Dot Diagrams Chemistry For Non Majors
Electron Dot Diagrams Chemistry For Non Majors
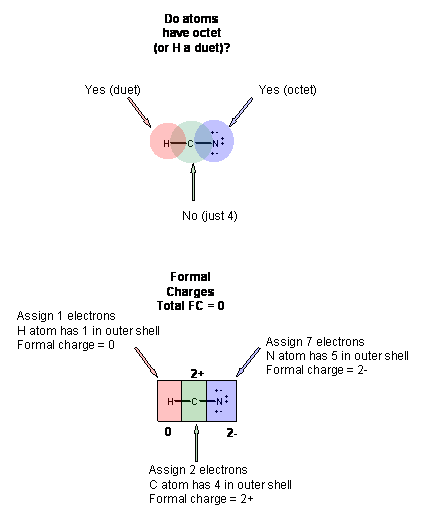
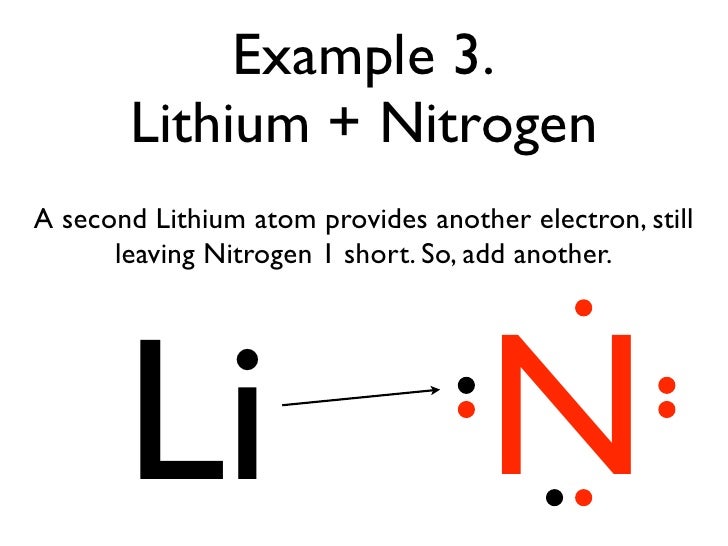
0 Response to "Lewis Dot Diagram For N"
Post a Comment