Be2 Molecular Orbital Diagram
Register alias and password only available to students enrolled in dr. A draw the molecular orbital diagram.
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
Turning to be2 we have be2 5 valence es.
Be2 molecular orbital diagram. Now we know that in a molecule the atomic orbitals overlap to form molecular orbitals which are bonding antibonding and in some cases a nonbonding molecular orbital results. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. The electronic configuration of bez 4 is.
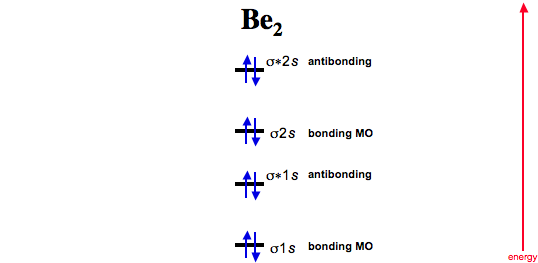
The electrons in each atomic orbital are represented by arrows. Bonding order is 0 meaning it does not bond and it is diamagnetic. Number of valence electrons in be atom 2 thus in the formation of be 2 molecule two outer electrons of each be atom ie.
For the ion be2. The energy of the bonding molecular orbital is less than the energy of the atomic orbital whereas the energy of the antibonding orbital is higher than that of the atomic orbital and it tries to destabilise the molecule. In general bonding molecular orbitals are lower in energy than either of their parent atomic orbitals.
D write the electron configuration of the ion. B calculate the bond order. Dashed lines connect the parent atomic orbitals with the daughter molecular orbitals.
In the middle of the diagram the molecular orbitals of the molecule of interest are written. 4 be 1s 2 2s 1 be 2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.
The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined. Molecular orbital diagram for beryllium dimer be2 fill from the bottom up with 4 electrons total. σ12e σ22e π11e σ30e π20e σ4e bond order ½σ bonding e σ antibonding e so if we take the bonding effect σ2 2e as not quite 1 π11e as 05 then the bond order of be2 is estimated to be 05 bo 10 ie bound.
C would this ion exist. And these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. 4 in all have to be accommodated in various molecular orbitals in the increasing order of their energies.
Be2 Molecular Orbital Diagram 74172 Enews
 Mot Molecular Orbital Diagrams For Li2 Li2 Be2 B2 C2 N2 Youtube
Mot Molecular Orbital Diagrams For Li2 Li2 Be2 B2 C2 N2 Youtube
 Orbitals What Is The Origin Of The Differences Between The Mo
Orbitals What Is The Origin Of The Differences Between The Mo
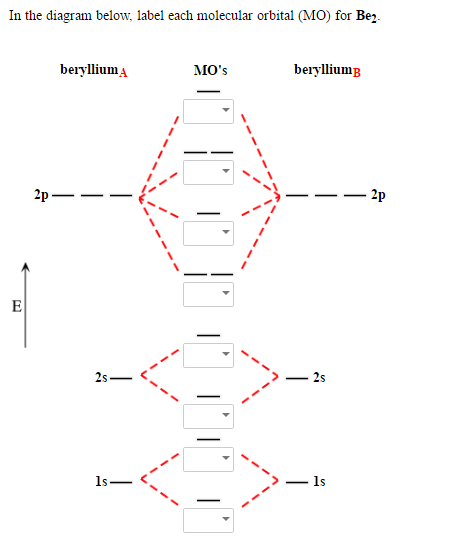 Solved In The Diagram Below Label Each Molecular Orbital
Solved In The Diagram Below Label Each Molecular Orbital
B2 Molecular Orbital Diagram For 2 Free Wiring Diagram For You
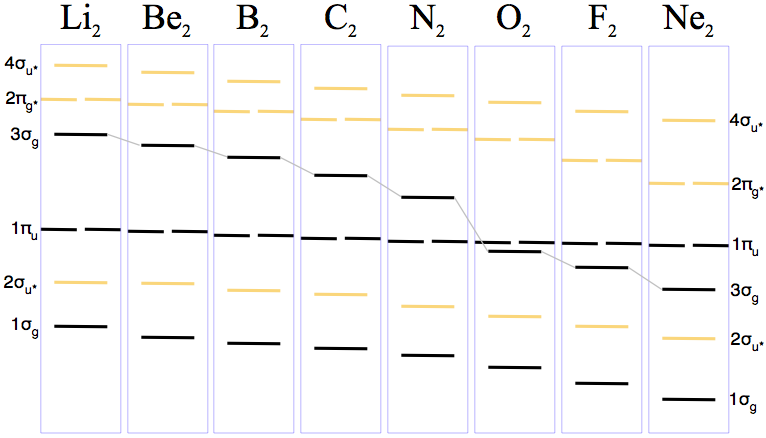
 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
Be2 Molecular Orbital Diagram Best Of How To Draw Orbital Diagrams
Activity 201 9 Molecular Orbital Theory
 Molecular Orbital Theory Build Be2 Youtube
Molecular Orbital Theory Build Be2 Youtube
 Be2 Molecular Orbital Diagram Recent Progress In Two Dimensional
Be2 Molecular Orbital Diagram Recent Progress In Two Dimensional
B2 Molecular Orbital Diagram Best Wiring Library
Be2 Molecular Orbital Diagram Best Of How To Draw Orbital Diagrams
 Oxygen Molecular Orbital Diagram Explanation Free Wiring Diagram
Oxygen Molecular Orbital Diagram Explanation Free Wiring Diagram
Be2 Molecular Orbital Diagram Best Of How To Draw Orbital Diagrams
Be2 Molecular Orbital Diagram New Diatomic Species Mo Theory
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 Pictorial Molecular Orbital Theory Chemistry Libretexts
Pictorial Molecular Orbital Theory Chemistry Libretexts
 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 Molecular Orbital Theory Build Be2 Youtube
Molecular Orbital Theory Build Be2 Youtube
.png) Be2 Molecular Orbital Diagram 3626 Loadtve
Be2 Molecular Orbital Diagram 3626 Loadtve
B2 Molecular Orbital Diagram Best Wiring Library

0 Response to "Be2 Molecular Orbital Diagram"
Post a Comment