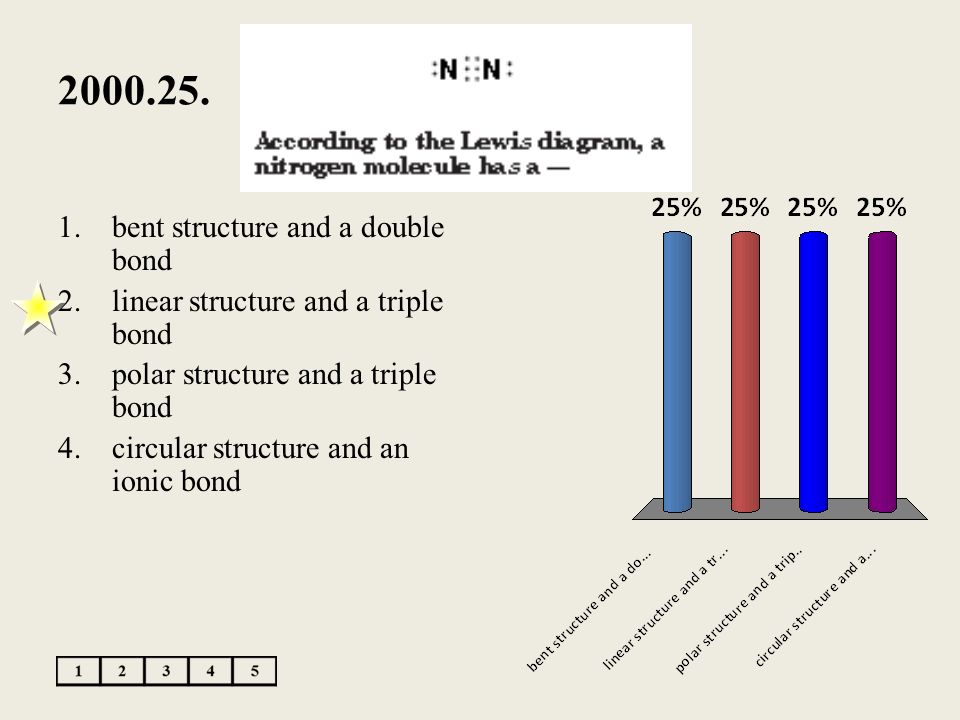
According To The Lewis Diagram A Nitrogen Molecule Has A
Asf5 however has a triangular bipyramidal shape. Which of the following is an acceptable lewis structure for chloromethane ch3cl.
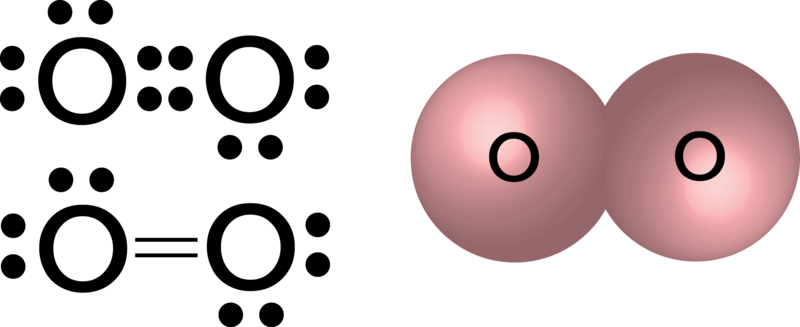
Which of the following is an acceptable lewis structure for the diatomic nitrogen molecule.
:max_bytes(150000):strip_icc()/what-are-the-seven-diatomic-elements-606623-v3-5b562dab46e0fb0037fee8c7.png)
According to the lewis diagram a nitrogen molecule has a. Bent structure and a double bond. 13 according to their placement on the periodic table which elements would have the most similar atomic structures. According to the lewis diagram a nitrogen molecule has a linear structure and a triple bond if the temperature of a reaction is increased the reaction proceeds at a much quicker rate because the.
It is a triangular pyramid with as having an unbonded electron pair. Circular structure and an ionic bond. Sodium iodide exhibits what type of bond.
It also is a good example of a molecule with a triple bond. Circular structure and an ionic bond 2. Nitrogen molecule has a a.
Drawing the lewis structure for n 2 dinitogen or nitrogen gas nitrogen n 2 is a commonly tested lewis structure due to its importance on earth about 78 of the earths atomsphere is n 2. According to the periodic table mg will most likely react with elements in which of these groups. Polar structure and a triple bond d.
There are 10 valence electrons available for the lewis structure for n 2. Bent structure and a double bond b. Learn vocabulary terms and more with flashcards games and other study tools.
Polar structure and a triple bond. According to the lewis diagram a. According to the lewis diagram to the right a nitrogen molecule has a a.
Linear structure and a triple bond c. According to the lewis diagram nitrogen molecule has a. According to the honc rule how many covalent bonds form around oxygen.
Cobalt a transition metal can have an. Provide students an opportunity to arrange elements in order of decreasing reactivity using the periodic table. A sodiumandscandium b.
Linear structure and a triple bond c. Oxidation number of either 2 or 3. In the same group and to identify elements for similar atomic structure.
Start studying chemistry sol practice questions. You would expect the molecule to be polar because of that unbonded pair. Asf3 has the same lewis structure as nh3.
 Atoms Molecules And Compounds Manoa Hawaii Edu
Atoms Molecules And Compounds Manoa Hawaii Edu
Lesson 9 Polarity Simple Cases
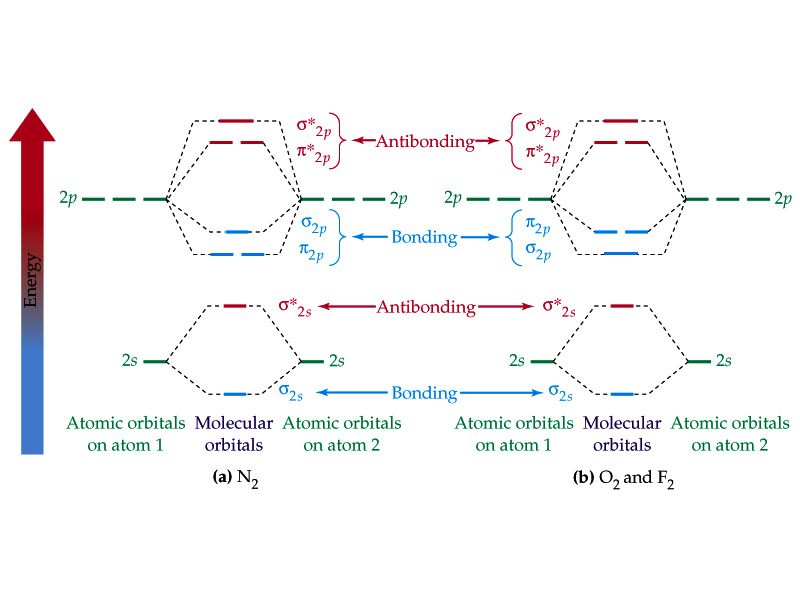
Introduction To Molecular Orbital Theory
Cyanide Molecule The Two Concentric Semicircles In The Lewis
 Co Electron Dot Diagram Wiring Diagram Database
Co Electron Dot Diagram Wiring Diagram Database
 Chemistry Nomenclature Chemical Formulas And Reactions Released
Chemistry Nomenclature Chemical Formulas And Reactions Released
 The Image Below Shows Two Nitrogen Atoms For These Two Atoms To Form
The Image Below Shows Two Nitrogen Atoms For These Two Atoms To Form
 Chapter 07 Html Chm2045 F13 All
Chapter 07 Html Chm2045 F13 All
:max_bytes(150000):strip_icc()/what-are-the-seven-diatomic-elements-606623-v3-5b562dab46e0fb0037fee8c7.png) What Are The 7 Diatomic Elements
What Are The 7 Diatomic Elements

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
Bond Angles And The Shapes Of Molecules
 What Is The Lewis Structure Of N2 Socratic
What Is The Lewis Structure Of N2 Socratic
Sparknotes Atomic Structure Electron Configuration And Valence
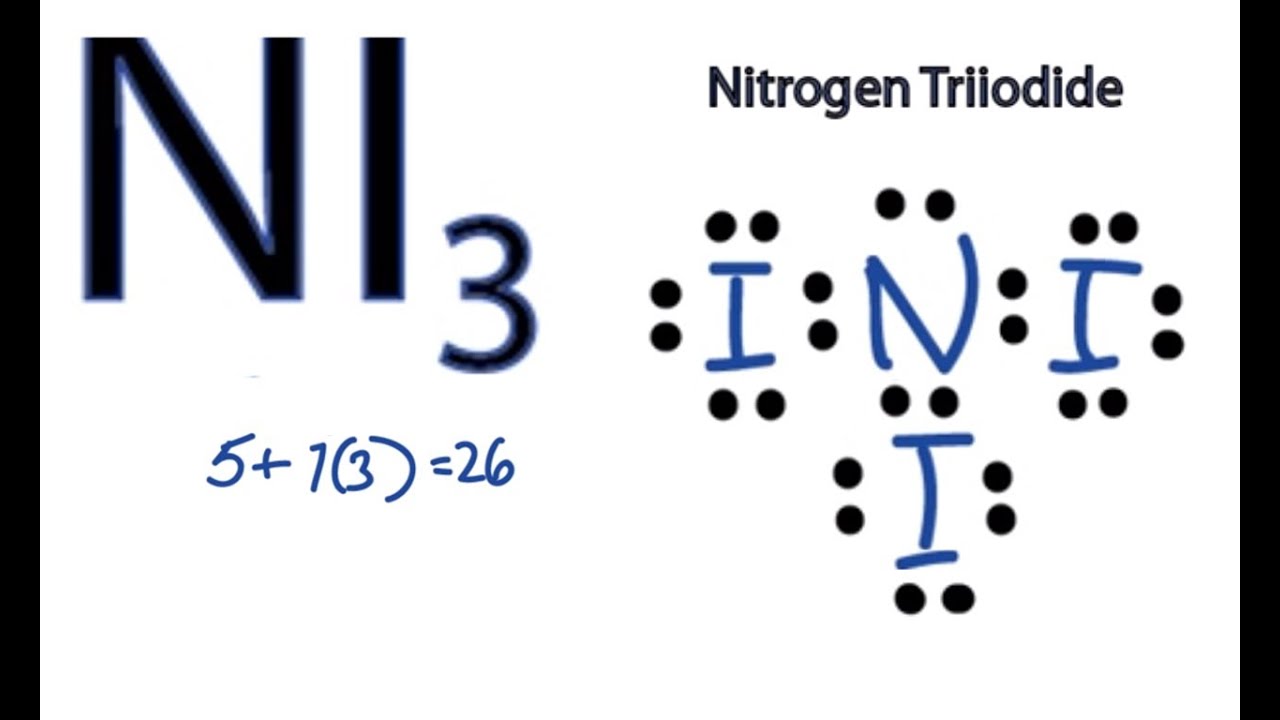 Ni3 Lewis Structure How To Draw The Dot Structure For Ni3
Ni3 Lewis Structure How To Draw The Dot Structure For Ni3
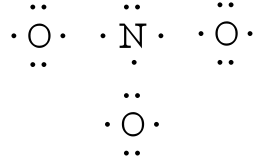
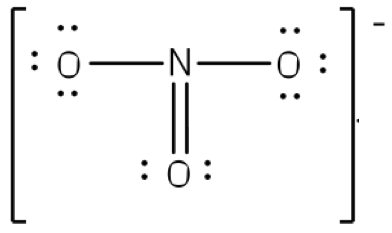
0 Response to "According To The Lewis Diagram A Nitrogen Molecule Has A"
Post a Comment