Molecular Orbital Energy Level Diagram
Two atomic orbitals in phase create a larger electron density which leads to the σ orbital. Electrons are added to molecular orbitals one at a time starting with the lowest energy molecular orbital.
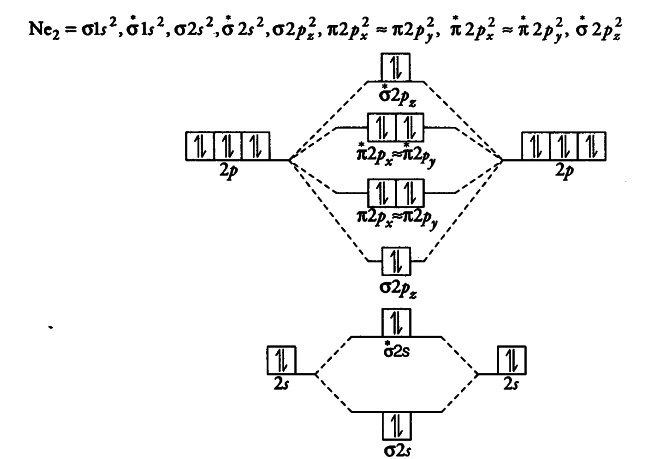 Use The Molecular Orbital Energy Level Diagram To Show That Cbse
Use The Molecular Orbital Energy Level Diagram To Show That Cbse
The spatial distribution and energy of one or one pair of electrons.
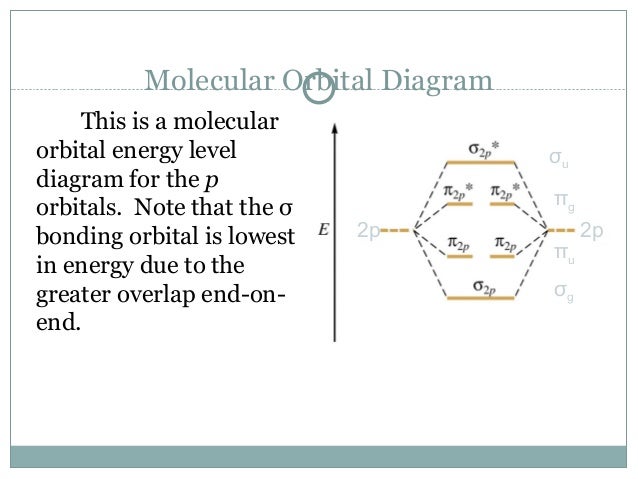
Molecular orbital energy level diagram. The superposition of the two 1s atomic orbitals leads to the formation of the σ and σ molecular orbitals. Thus we can see that combining the six 2 p atomic orbitals results in three bonding orbitals one σ and two π and three antibonding orbitals one σ and two π. In the middle of the diagram the molecular orbitals of the molecule of interest are written.
Most commonly a mo is represented as a linear combination of atomic orbitals the lcao mo method especially in qualitative or very approximate usage. There are two mo diagrams you need to memorize for diatoms n2 o2 ne2 etc. Dashed lines connect the parent atomic orbitals with the.
For each pair of atomic orbitals that combine one lower energy bonding molecular orbital and one higher energy antibonding orbital result. Because the energy of the two electrons is lower than the energy of the individual atoms the molecule is stable. If the two 1s orbitals are not in phase a node between them causes a jump in energy the σ orbital.
B4 and results in an increased concentration of electrons between the two nuclei it is a bonding molecular orbital and has a lower energy than the original atomic orbitals. For molecules li 2 be 2 b 2 c 2 and n 2 the molecular orbital energy level diagram in the diagram the molecular orbitals are place at the center and the atomic orbitals at the same level. The two electrons associated with a pair of hydrogen atoms are placed in the lowest energy or bonding molecular orbital as shown in the figure below.
A molecular orbital can specify the electron configuration of a molecule. Practice energy diagrams for molecular orbital theory. Thus for diatomic molecules of second period li 2 to ne 2 there are two types of energy levels of mos.
B the shapes of the molecular orbitals are obtained by squaring the wave functions for mo1 and mo2. Explain energy level diagram for molecular orbitals. Calculate the number of bonding and antibonding electrons in simple molecules.
One is for the elements up to nitrogen. A the molecular orbital energy level diagram for the h2 molecule. Molecular orbital diagrams of diatomic molecules.
Because the s molecular orbital is the sum of two atomic orbitals 1 22. The other is for after nitrogen starting at oxygen.
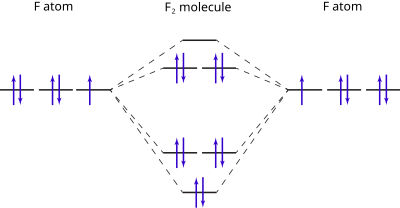 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 Molecular Orbital Theory General Chemistry Lecture 1140 Dr
Molecular Orbital Theory General Chemistry Lecture 1140 Dr
 10 5 Molecular Orbital Theory Chemistry Libretexts
10 5 Molecular Orbital Theory Chemistry Libretexts
Explaining The Geometry Of Simple Molecules Using Molecular Orbital
 Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack
Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack
 Solved According To The Molecular Orbital Energy Level Di
Solved According To The Molecular Orbital Energy Level Di
 Molecular Orbital Energy Level Diagram Britannica Com
Molecular Orbital Energy Level Diagram Britannica Com
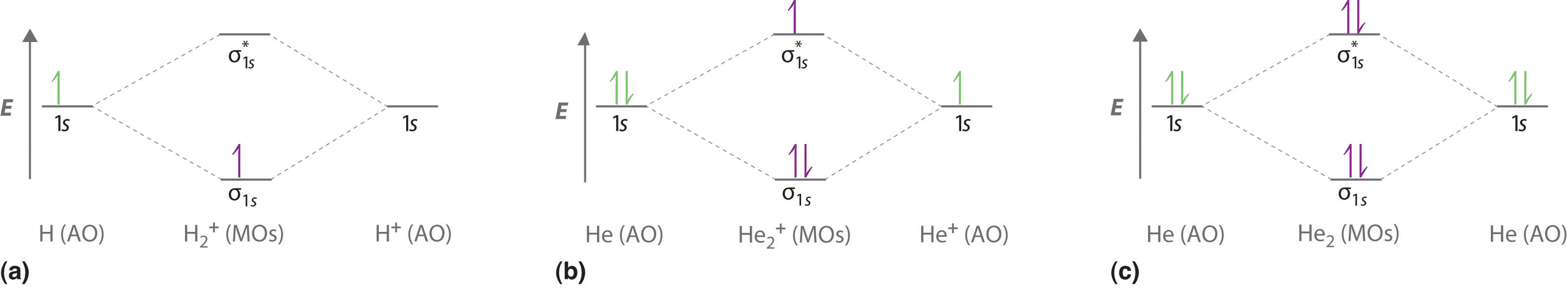 10 5 Molecular Orbital Theory Chemistry Libretexts
10 5 Molecular Orbital Theory Chemistry Libretexts
Introduction To Molecular Orbital Theory
 What Is The Energy Level Diagram Of N2 And F2 Brainly In
What Is The Energy Level Diagram Of N2 And F2 Brainly In
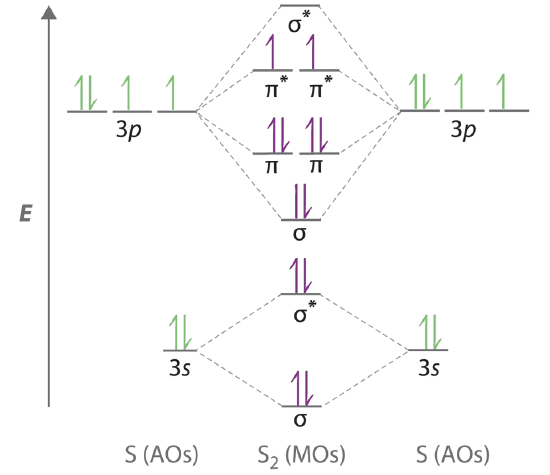 9 8 M O Theory And The Period 2 Diatomic Molecules Chemistry
9 8 M O Theory And The Period 2 Diatomic Molecules Chemistry
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
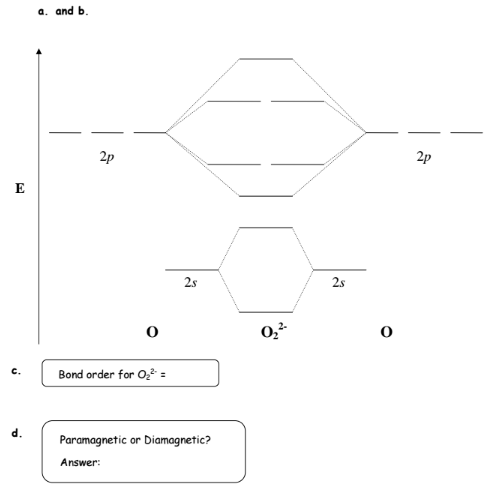 Solution Complete The Mo Energy Level Dia Clutch Prep
Solution Complete The Mo Energy Level Dia Clutch Prep
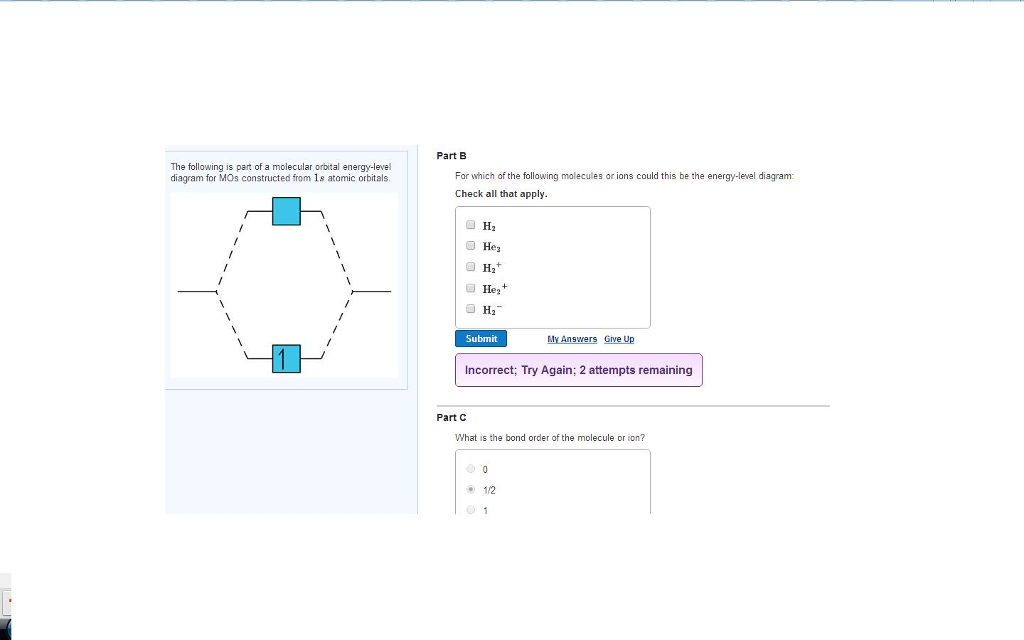 Solved The Following Is Part Of A Molecular Orbital Energ
Solved The Following Is Part Of A Molecular Orbital Energ
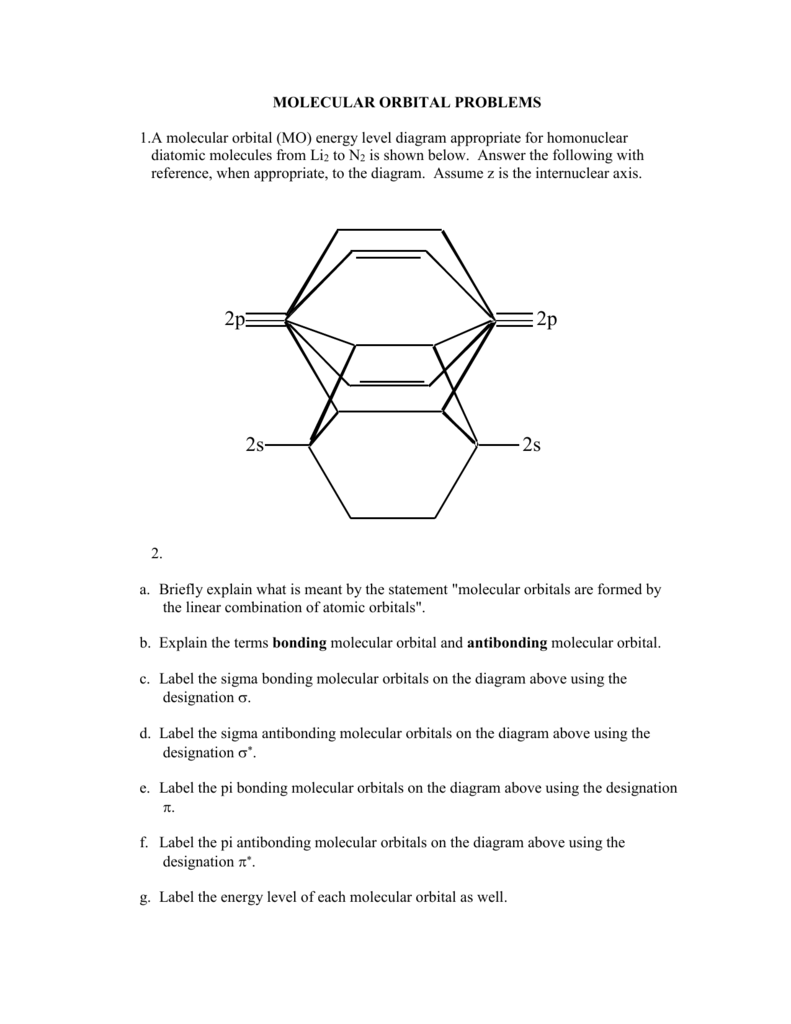 A Molecular Orbital Mo Energy Level Diagram Parkway C 2
A Molecular Orbital Mo Energy Level Diagram Parkway C 2
Hf Molecular Orbital Diagram Orbital Energy Level Diagram
 A 1 Molecular Orbital Theory Chemistry Libretexts
A 1 Molecular Orbital Theory Chemistry Libretexts
 File Ammonia Mo Diagram Jpg Wikimedia Commons
File Ammonia Mo Diagram Jpg Wikimedia Commons
 Energy Level Diagram For Molecular Orbitals Chemical Bonding And
Energy Level Diagram For Molecular Orbitals Chemical Bonding And
 Constructing The O2 Molecular Orbital Energy Level Diagram Youtube
Constructing The O2 Molecular Orbital Energy Level Diagram Youtube
0 Response to "Molecular Orbital Energy Level Diagram"
Post a Comment