The Diagram Represents A Spontaneous Reaction Use The Diagram To Answer The Questions Below
2789184 home questions sciencemath chemistry chemistry others the diagram represents a spontaneous reaction. Use the diagram to answer the questions below.
Energy And Enzymes Biology 1510 Biological Principles
Use the following potential energy diagram to answer the questions below.
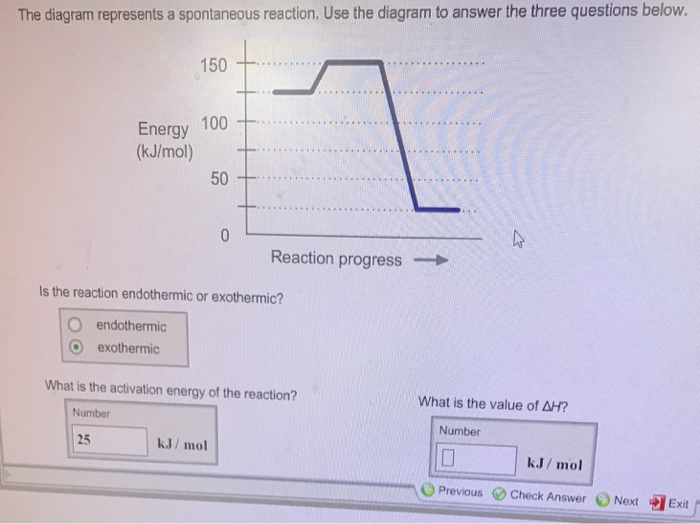
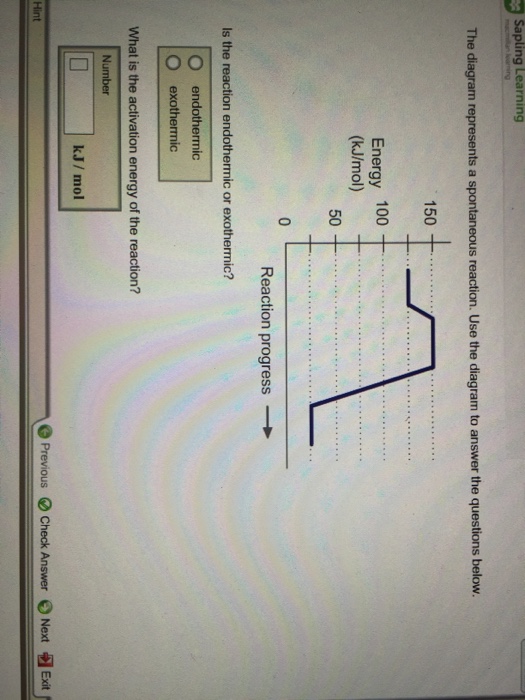
The diagram represents a spontaneous reaction use the diagram to answer the questions below. Kj b determine the activation energy for the reverse reaction. Note that just because a reaction is called spontaneous does not necessarily mean that the reaction will happen instantly or quickly. B the endpoint occurs when equal moles of substances react.
T c the equivalence point occurs when equal moles of substances react. D the equivalence point occurs when the indicator changes color. Is the reaction endothermic or exothermic.
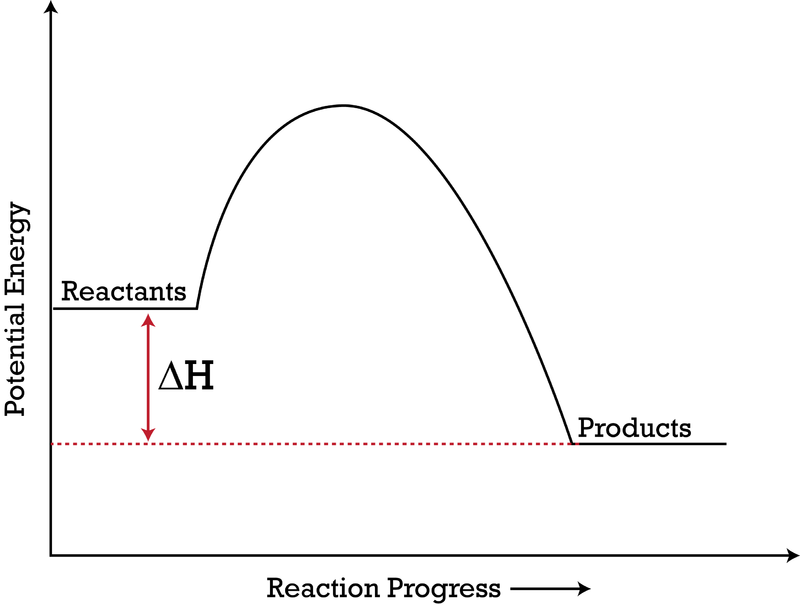
Endothermic exothermic what is the activation energy of the reaction. Use the diagram to answer the questions belowis the reaction endothermic or exothermic. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled.
100 80 60 40 20 0 progress of reaction a determine the activation energy for the forward reaction. Kj c what is the enthalpy change h for the forward reaction. A spontaneous reaction is a chemical reaction that will occur because of the nature of the system once it is initiated.
This state is also known as an activated complex. Can be found with the difference between the potential energies of the reactants and products. Which information about a chemical reaction is provided by a potential energy diagram.
Is the reaction endothermic or exothermic. A the endpoint is the same as the equivalence point. Find an answer to your question use the diagram below for questions 28 and 29.
Although we can write any chemical reaction on paper not all chemical reactions will occur. Potential energy diagram worksheet 2. Use the diagram to answer the questions belowis the reaction endothermic or exothermic.
Show transcribed image text the diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Endothermic exothermic what is the activation energy of the reaction.
At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products. In the diagram above which symbol represents the light bulb. Show transcribed image text the diagram represents a reaction.

 Solved The Diagram Represents A Spontaneous Reaction Use
Solved The Diagram Represents A Spontaneous Reaction Use
 Energy Profile Chemistry Wikipedia
Energy Profile Chemistry Wikipedia
Name Chemistry Regents August 2015
 Energy Profile Chemistry Wikipedia
Energy Profile Chemistry Wikipedia
 Physicists Provide Support For Retrocausal Quantum Theory In Which
Physicists Provide Support For Retrocausal Quantum Theory In Which
 Estimating Moisture Content Of Landfilled Municipal Solid Waste
Estimating Moisture Content Of Landfilled Municipal Solid Waste
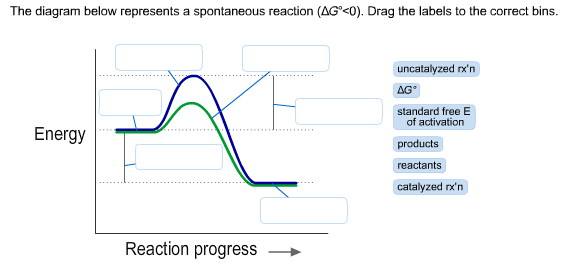 Solved The Diagram Below Represents A Spontaneous Reactio
Solved The Diagram Below Represents A Spontaneous Reactio
 Mcat General Chemistry Review Summary Gold Standard Mcat Prep
Mcat General Chemistry Review Summary Gold Standard Mcat Prep
 Exothermic And Endothermic Reactions
Exothermic And Endothermic Reactions
Test1 Ch15 Kinetics Practice Problems
 Electrochemical Reaction Chemistry Britannica Com
Electrochemical Reaction Chemistry Britannica Com
Entropy And The 2nd 3rd Laws Of Thermodynamics


0 Response to "The Diagram Represents A Spontaneous Reaction Use The Diagram To Answer The Questions Below"
Post a Comment