Temperature Vs Composition Phase Diagram
All you have to do is to use the liquid composition curve to find the boiling point of the liquid and then look at what the vapour composition would be at that temperature. These are phase diagrams which show the composition of two phases in equilibrium at a given pressure and how these compositions change with temperature as opposed to the pressure composition diagrams which showed the pressure dependence of the composition at a fixed temperature.
Lecture 3 Solutions Activities And Phase Diagrams
B diagram and that of the liquid by point 5 on the same diagram.
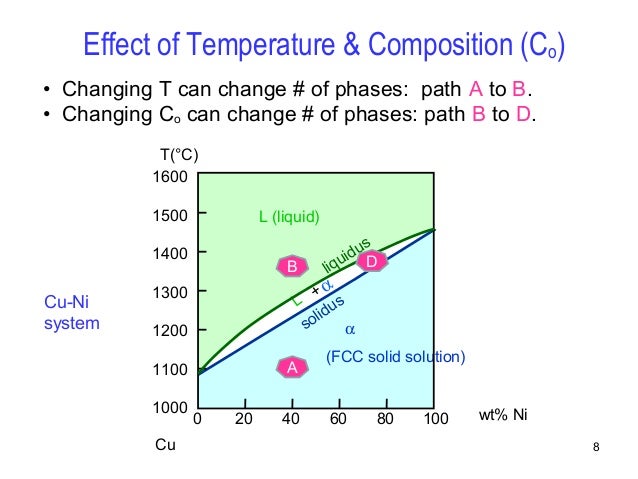
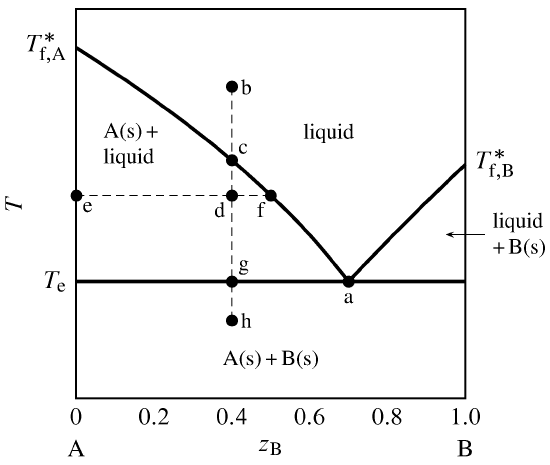
Temperature vs composition phase diagram. No driving force for change. The system can in fact lower its free energy even further by splitting up into a solid of composition x. Phase diagrams can use other variables in addition to or in place of temperature pressure and composition for example the strength of an applied electrical or magnetic field and they can also involve substances that take on more than just three states of matter.
This video is unavailable. Specifically in this animation we consider a system that shows only the existence of a single phase or two phases at any temperature and composition. Ms15a gibbs free energy and phase diagrams 1100.
Phase region we have a vapor phase and a liquid phase in equilibrium with each other. Thus a point in this region must correspond to two different compositions. So the phase diagram is a plot of composition vs.
Where this isotherm intersects the solidus at point b the composition of the solid can be found by drawing a vertical line to the base of the diagram. Suppose the total composition were 5050. The gibbs free energy of the solid is given by point 4 on the gx.
Lecture 6 9 phase diagrams. Temperature with the equilibrium phases marked at different combinations of these two parameters. L b shown on both diagrams.
The traces in the phase diagrams separate whenever the composition of the vapor differs from the composition of the liquid at the same temperature. If physical conditions change system is no longer in equilm and must shift type or proportions of the minerals ie cooling of magma body. For example in the next diagram if you boil a liquid mixture c 1 it will boil at a temperature t 1 and the vapour over the top of the boiling liquid will have the composition c 2.
System in equilibrium. Ie the t p and proportions of minerals and melt remain fixed. Skip navigation sign in.
The composition of this plagioclase can be found by drawing an isotherm line of constant temperature a horizontal line in this diagram through the temperature 1410 o. And a liquid of composition x. You could make this composition using 50 of 5050 vapor and 50 of 5050 liquid but you could also make it from 8333 of 4555 vapor and 1667 of 7525 liquid as well as from many other combinations.
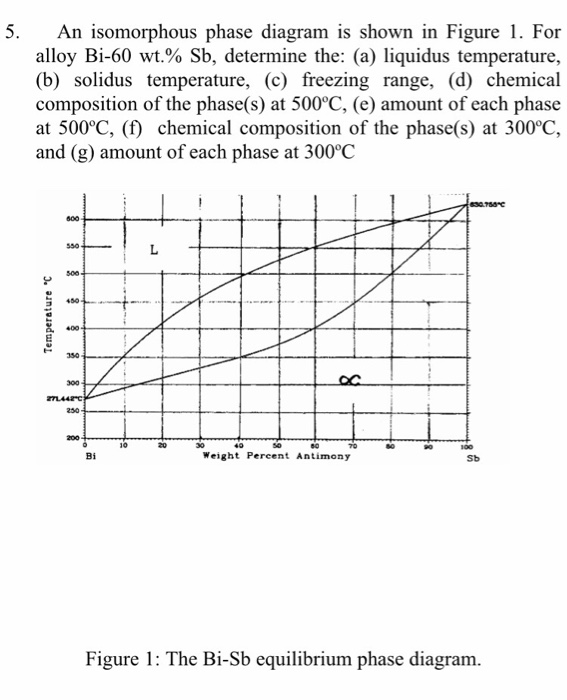
 Calculated Temperature Vs Composition Phase Diagram At Different
Calculated Temperature Vs Composition Phase Diagram At Different
 The Application Of Temperature Composition Phase Diagrams For Hot
The Application Of Temperature Composition Phase Diagrams For Hot
Phase Composition Diagram From Eric Weisstein S World Of Chemistry
Phase Diagrams Of Unary And Binary Systems Ppt Video Online Download

 Please Sketch A Temperature Vs Composition Phase D Chegg Com
Please Sketch A Temperature Vs Composition Phase D Chegg Com
Phase Transformations And Phase Diagrams Substech
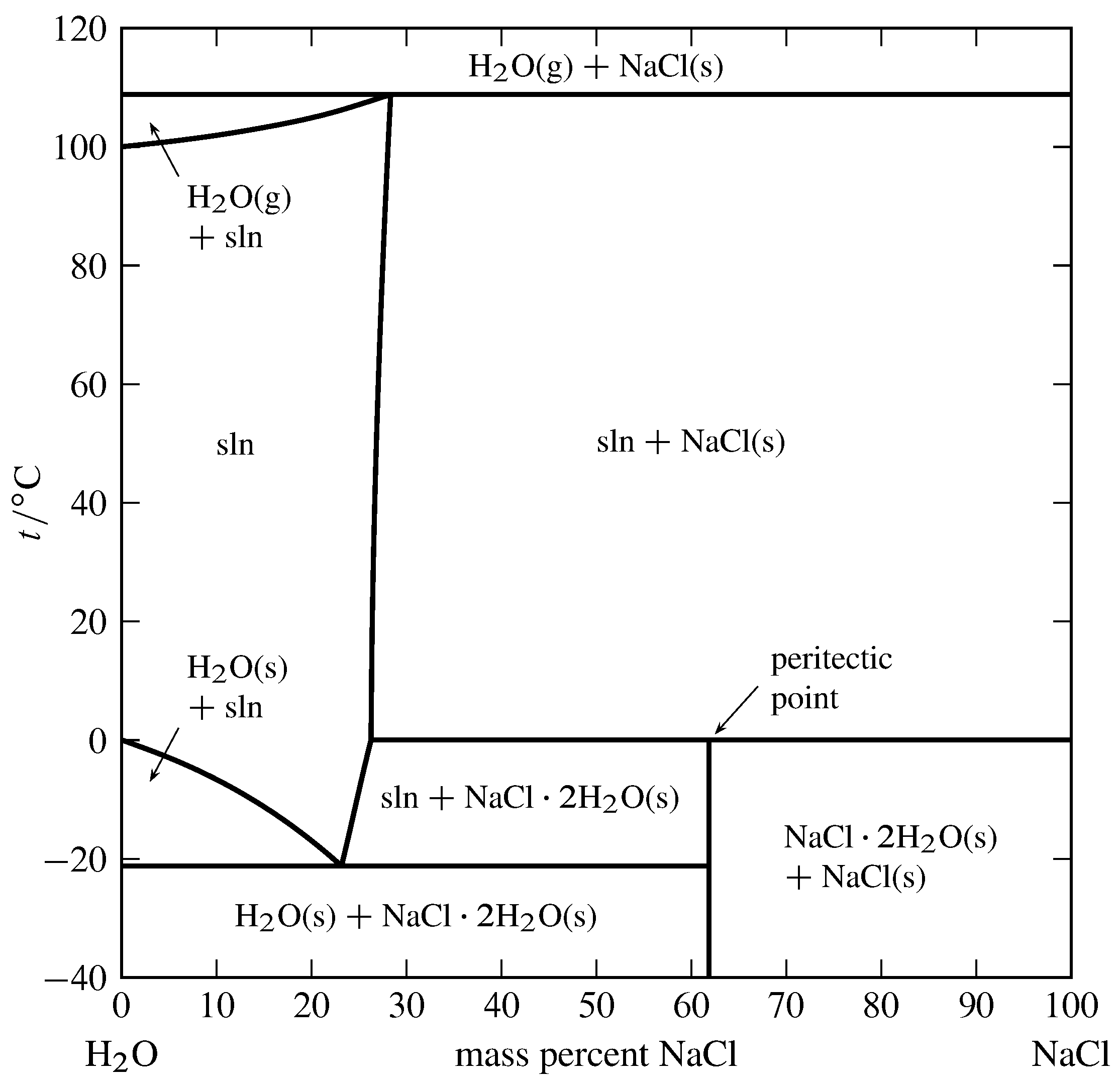 13 2 Phase Diagrams Binary Systems Chemistry Libretexts
13 2 Phase Diagrams Binary Systems Chemistry Libretexts
Practical Maintenance Blog Archive Phase Diagrams Part 1
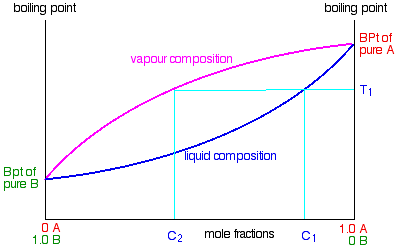 Raoult S Law And Ideal Mixtures Of Liquids
Raoult S Law And Ideal Mixtures Of Liquids
 Chem584 Phasediagrams Phase Diagrams Multiple Variables Can Be
Chem584 Phasediagrams Phase Diagrams Multiple Variables Can Be
 13 2 Phase Diagrams Binary Systems Chemistry Libretexts
13 2 Phase Diagrams Binary Systems Chemistry Libretexts
 Functionality Improvement Of Nimesulide By Eutectic Formation With
Functionality Improvement Of Nimesulide By Eutectic Formation With
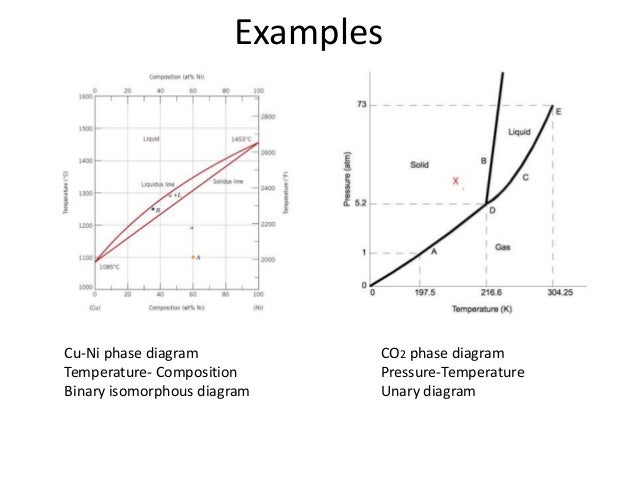

0 Response to "Temperature Vs Composition Phase Diagram"
Post a Comment