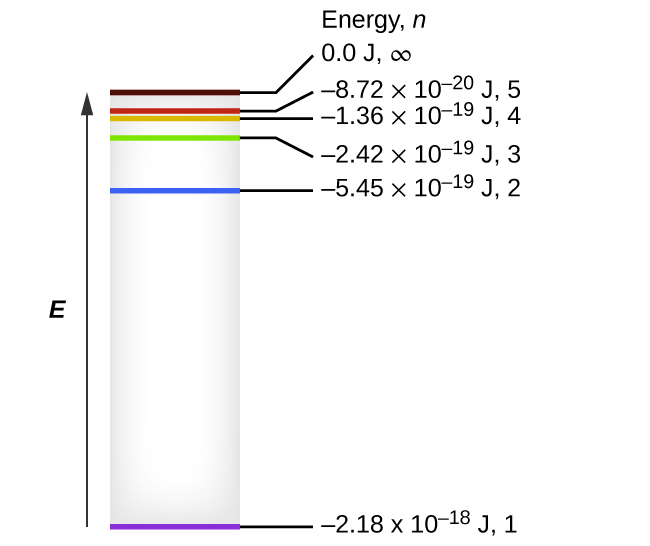
The Figure Is An Energy Level Diagram For A Simple Atom Figure 1
The figure is an energy level diagram for a quantum system. What wavelengths appear in the atoms em.
 High School Chemistry Atomic Size Wikibooks Open Books For An
High School Chemistry Atomic Size Wikibooks Open Books For An
Exercise 1 description.
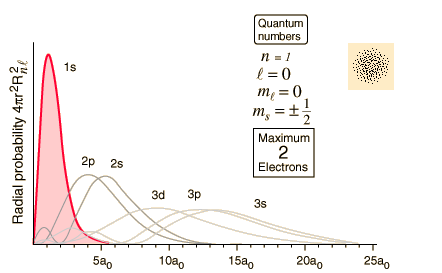
The figure is an energy level diagram for a simple atom figure 1. What wavelengths appear in the atoms emission spectrum. What wavelengths in nm app. The figure figure 1 is an energy level diagram for a simple atom.
Biology8 chapter 6 pratice test. Figure 1 if an electron at level 1 in a hydrogen atom absorbs 102 ev of energy it moves to level 2. Answer in nm 1 following.
From wavelength 2 to 1. The answer to figure p298 is an energy level diagram for a simple atom. The figure is an energy level diagram for a simple atom.
Five poss election transitions are indicated labeled a through e. What wavelengths appear in the atoms emission spectrum. What wavelengths appear in the atoms absorption spectrum.
The allowed energies of a simple atom are 00 ev 40 ev and 60 ev. I an electron with 20 ev of figure p298 kinetic energy collides with the in class 40 ev 830 nm 500 nm 310 nm 15 ev 830 nm 310 nm 00 ev 2 only 050 ev. What wavelengths appear in the systems emission spectrum.
Released because the energy level of the reactants is greater than that of the products. Consider the energy diagram for a chemical reaction in figure 6 3. The labeled transitions a through e represent an electron moving between energy levels.
The figure is an energy level diagram for a quantum system. I figure p298 is an energy level diagram for a simple atom. The figure below represents an energy level diagram for a fictitious atom.
What wavelengths appear in the atoms a emission spectrum and b absorption spectrum. What wavelengths in nm app. Answer in nm b.
From wavelength 3 to 2. The following diagram represents energy levels in a hydrogen atom. Overall is energy released or absorbed.
The figure figure 1 is an energy level diagram for a simple atom. What wavelengths appear in the atoms a emission spectrum and b absorption spectrum is broken down into a number of easy to follow steps and 24 words. From wavelength 3 to 1.
The figure is an energy level diagram for a simple atom. What wavelengths appear in the atoms em. The figure is an energy level diagram for a simple atom.

 Chemistry I Atoms And Molecules
Chemistry I Atoms And Molecules
Module 1 Atomic Structure Lecture 1 Structural Chemistry
Figure P29 8 Is An Energy Level Diagram For A Simple Atom Studysoup
 Electronic Configuration The Atom Siyavula
Electronic Configuration The Atom Siyavula
 The Trouble With The Aufbau Principle Feature Education In Chemistry
The Trouble With The Aufbau Principle Feature Education In Chemistry
 Electronic Configuration The Atom Siyavula
Electronic Configuration The Atom Siyavula
 Basic Schematic Of A Typical Atomic Clock Referenced In This Paper
Basic Schematic Of A Typical Atomic Clock Referenced In This Paper
 Spectroscopy Energy States Of Real Diatomic Molecules Britannica Com
Spectroscopy Energy States Of Real Diatomic Molecules Britannica Com
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
 What Is Electricity Learn Sparkfun Com
What Is Electricity Learn Sparkfun Com
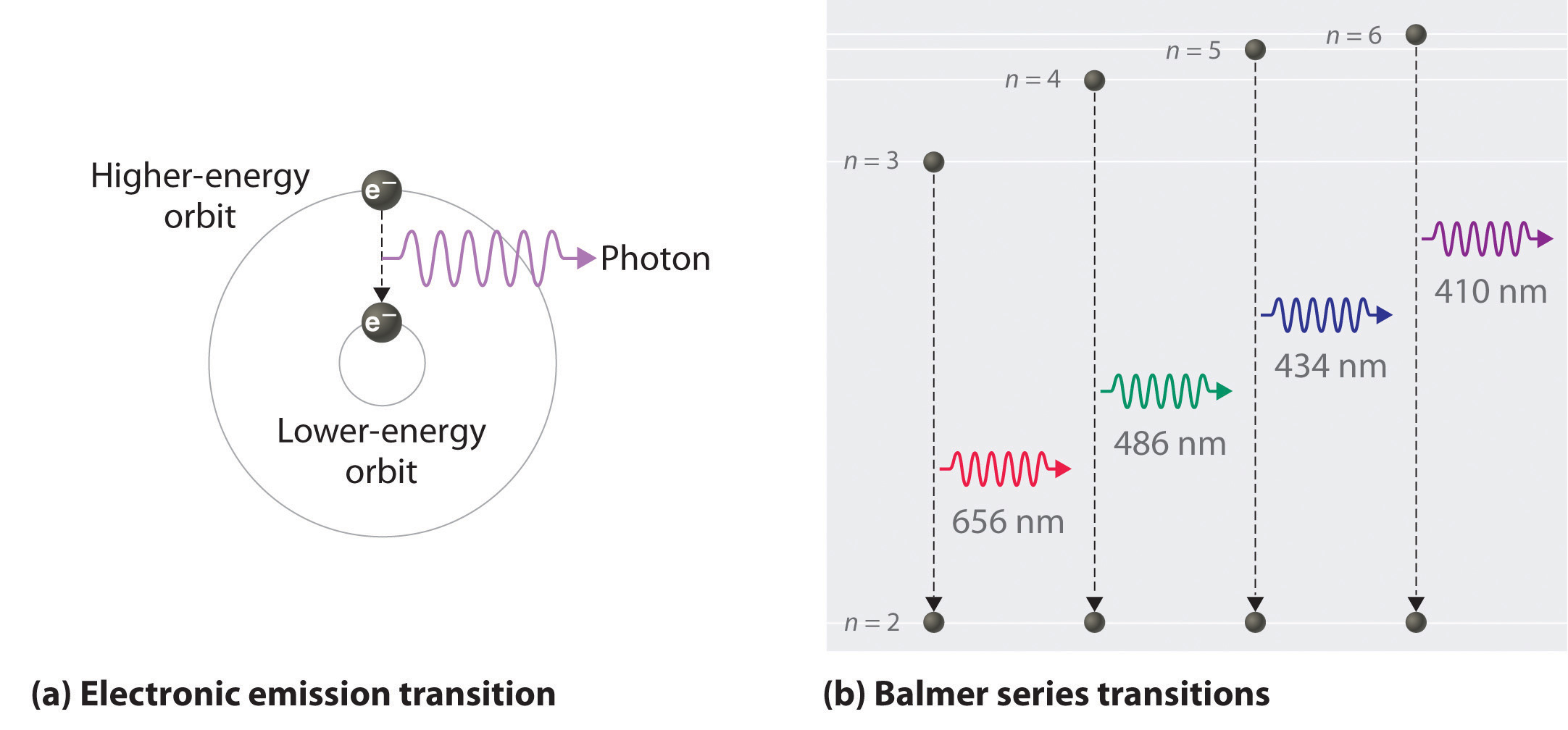 6 3 Line Spectra And The Bohr Model Chemistry Libretexts
6 3 Line Spectra And The Bohr Model Chemistry Libretexts
 Drawing Electron Configuration Diagrams Chemistry For All
Drawing Electron Configuration Diagrams Chemistry For All

0 Response to "The Figure Is An Energy Level Diagram For A Simple Atom Figure 1"
Post a Comment