Electron Dot Diagram For Lithium
A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Lithium fluoride lif 1.
Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6 1.
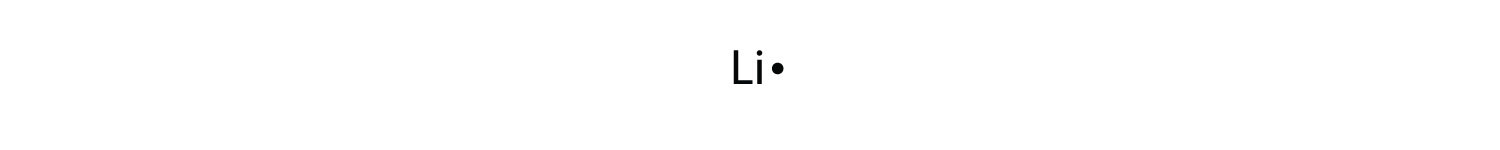
Electron dot diagram for lithium. Lithium fluoride compound can be represented as li or 1. I show you where lithium is on the periodic table and how to determine how many valence electrons it has. Choose the statement the correctly identifies the most stable of the elements.
The next atom lithium has an electron configuration of 1s 2 2s 1 so it has only one electron in its valence shell. Lithium is the most stable element because it has to lose only one electron to achieve a stable configuration. Lithium is the third element with a total of 3 electrons.
Lewis electron dot diagrams for ions have less for cations or more for. Its electron dot diagram resembles that of hydrogen except the symbol for lithium is used. The lewis structure electron dot diagram of each ion is used to construct the lewis structure electron dot diagram for the ionic compound.
Since 1s can only hold two electrons the remaining electron for li goes in the 2s orbital. Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6 1. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital.
The lewis structure electron dot diagram of each ion is used to construct the lewis structure electron dot diagram for the ionic compound. Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6 1. Fluorine atom gains one electron to form the anion f 3.
A step by step explanation of how to draw the lewis dot structure for li lithium. Beryllium has two valence electrons in its 2s shell. The lewis structure of a positive ion cation is positioned adjacent to the lewis structure of a negative ion anion.
Lithium atom loses one electron to form the cation li 2. Choose the statement that correctly identifies the most stable of the elements. In an electron dot diagram the symbol for an element is used to represent the nucleus and all nonvalence electrons.
The number of dots equals the number of valence electrons in the atom.
 Lewis Dot Notes Lewis Dot Diagrams Illustrates The Number Of
Lewis Dot Notes Lewis Dot Diagrams Illustrates The Number Of
Lewis Dot Diagram For Lithium Best Lewis Dot Structures And Ionic
Lewis Dot Diagram Iodine Inspirational Iodine U2013
 Exceptions To The Octet Rule Boundless Chemistry
Exceptions To The Octet Rule Boundless Chemistry
 Welcome Silently Begin Do Now 1 Describe How The Particles In A Gas
Welcome Silently Begin Do Now 1 Describe How The Particles In A Gas
Electron Dot Diagram For Lithium Fresh Dot Diagram Li Trusted Wiring
 Lewis Dot Structure For Lithium Li Youtube
Lewis Dot Structure For Lithium Li Youtube
 Ppt 1 How Many Total Electrons Are In A Neutral Atom Of Sulfur
Ppt 1 How Many Total Electrons Are In A Neutral Atom Of Sulfur
 Valence Electrons And The Periodic Table Youtube
Valence Electrons And The Periodic Table Youtube
Paramagnetism And Diamagnetism Video Electron Dot Diagram For
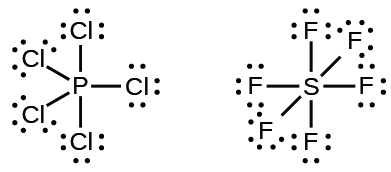 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
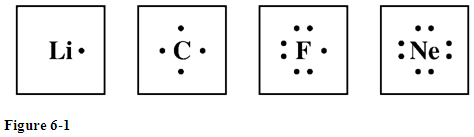 Solved Study The Electron Dot Diagrams For Lithium Carbo
Solved Study The Electron Dot Diagrams For Lithium Carbo
 Lithium Oxide Electron Dot Diagram Schematic Diagram
Lithium Oxide Electron Dot Diagram Schematic Diagram
Date 1916 Lewis Dot Diagram Worksheet E The Number Of Valance
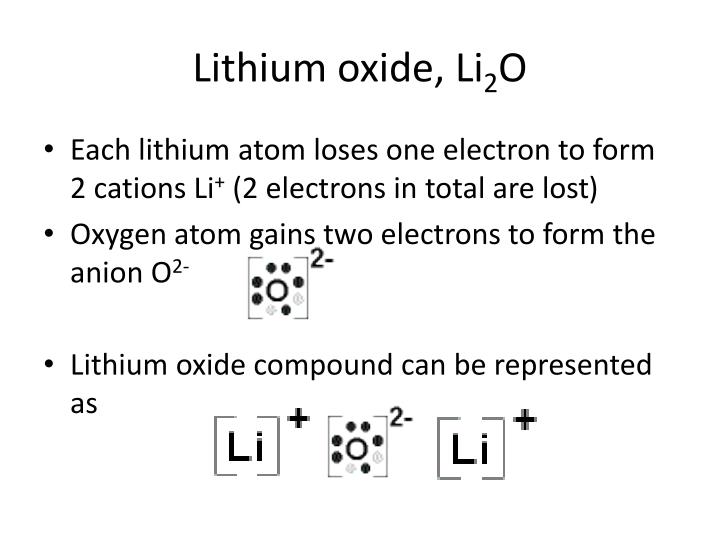 Ppt Lewis Structure And Bonding Powerpoint Presentation Id 5695002
Ppt Lewis Structure And Bonding Powerpoint Presentation Id 5695002
 A How Many Electrons Are In A Neutral Atom Of Lithium B How Many
A How Many Electrons Are In A Neutral Atom Of Lithium B How Many
Valence Dot Diagram Michaelhannan Co
Lewis Dot Diagram For Lithium Awesome Lithium Oxide Lewis Dot
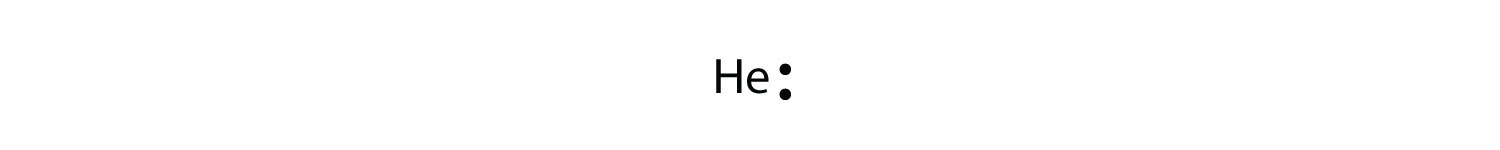

0 Response to "Electron Dot Diagram For Lithium"
Post a Comment