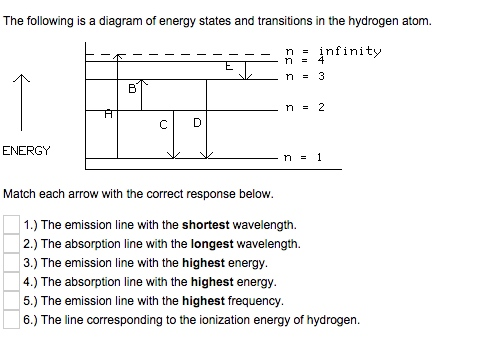
The Following Is A Diagram Of Energy States And Transitions In The Hydrogen Atom
Match each arrow with the correct response below. The following diagram represents energy levels in a hydrogen atom.
Answer Series 2 Answer Series 2 Answer The Transition Labeled
For a single electron instead of per mole the formula in ev electron volts is also widely used.

The following is a diagram of energy states and transitions in the hydrogen atom. 4 a the emission line with the shortest wavelength. The following is a diagram of energy states and transitions in the hydrogen atom. The ionization energy of an atom is the energy required to remove the electron completely from the atomtransition from ground state n 0 to infinity n.
The 2p level is split into a pair of lines by the spin orbit effect. 3 d the absorption line with the highest energy. E e0n2 where e0 136 ev 1 ev 160210 19 joules and n 123 and so on.
Hydrogen energy level plot. Match each of the responses below with the correct arrow from the figure. Figure 1 if an electron at level 1 in a hydrogen atom absorbs 102 ev of energy it moves to level 2.
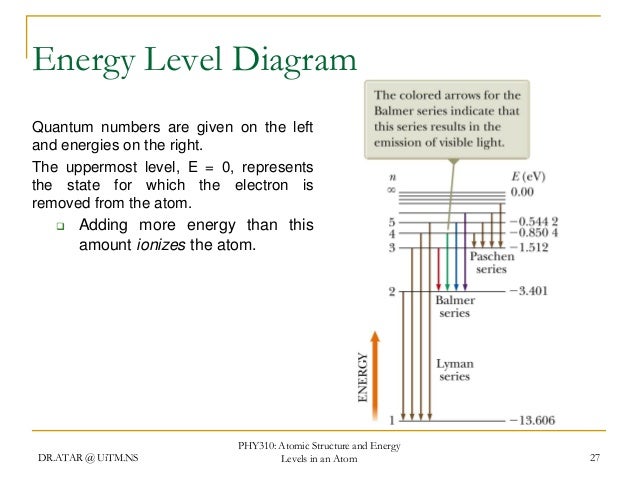
The energy level of the electron of a hydrogen atom is given by the following formula where n denotes the principal quantum number. The following is a diagram of energy states and transitions in the hydrogen atom. For hydrogen the ionization energy 136ev.
And even the 1s ground state is split by the interaction of electron spin and nuclear spin in what is called hyperfine structure. The 2s and 2p states are found to differ a small amount in what is called the lamb shift. Suppose that an electron in a hydrogen atom absorbs 102 ev of energy so that it moves from level 1 to level 2.
If none are correct enter none. Match each of the responses below with the correct arrow from the figure. 4 c the emission line with the highest energy.
The labeled transitions a through e represent an electron moving between energy levels. Its often helpful to draw a diagram showing the energy levels for the particular element youre interested in. The diagram for hydrogen is shown above.
1 b the absorption line with the longest wavelength. The labeled transitions a through e represent an electron moving between energy levels. The emission line with the highest energy.
B the electron returns to level 1 by emitting an ultraviolet photon with 102 ev of energy. The emission line with the shortest wavelength. Electron shells and energy levels.
The diagram represents energy levels in a hydrogen atom. The following is a diagram of energy states and transitions in the hydrogen atom. The n 1 state is known as the ground state while higher n states are known as excited states.
The energy is expressed as a negative number because it takes that much energy to unbind ionize the electron from the nucleus. The absorption line with the shortest wavelength. The formula defining the energy levels of a hydrogen atom are given by the equation.
When an excited electron returns to a lower level it loses an exact amount of energy by emitting a photon. Energy level diagrams and the hydrogen atom.
Relativistic Energy Levels For Hydrogen Atom Wolfram
How Can An Electron Leap Between Atomic Levels Without Passing
 Ib Chemistry Atomic Theory Wikibooks Open Books For An Open World
Ib Chemistry Atomic Theory Wikibooks Open Books For An Open World
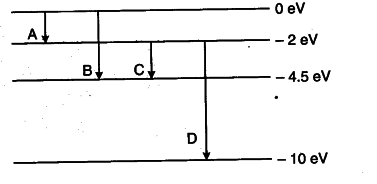 The Energy Levels Of A Hypothetical Atom Are Shown Below Cbse
The Energy Levels Of A Hypothetical Atom Are Shown Below Cbse
 Energy Levels Of Small Hydrogen Atom Level As A Function Of Orbital
Energy Levels Of Small Hydrogen Atom Level As A Function Of Orbital
 Transitions Between Two Levels Of The Small Hydrogen Atom As A
Transitions Between Two Levels Of The Small Hydrogen Atom As A
Chapter 6 Electronic Structure Of Atoms
A New Way To Explain The 511 Kev Signal From The Center Of The
 The Following Is A Diagram Of Energy States And Tr Chegg Com
The Following Is A Diagram Of Energy States And Tr Chegg Com
Techniques And Information Content Spectroscopy
Hydrogen Atom Energy Levels And Transitions
Hydrogen Atom For Each Of The Following Electronic Transitions In
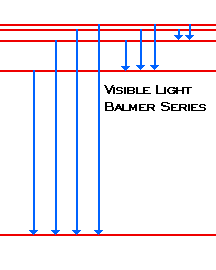
 Hydrogen Spectral Series Wikipedia
Hydrogen Spectral Series Wikipedia


0 Response to "The Following Is A Diagram Of Energy States And Transitions In The Hydrogen Atom"
Post a Comment